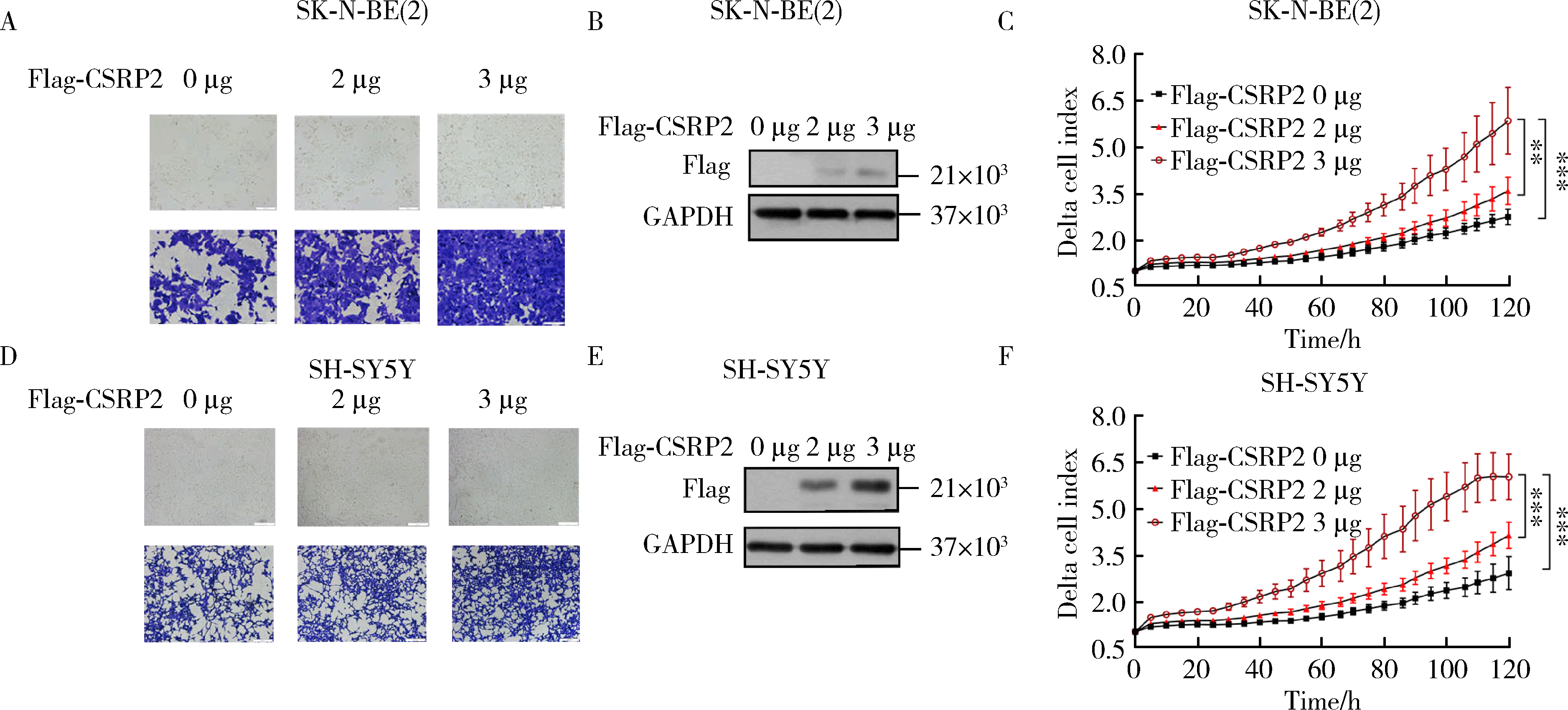
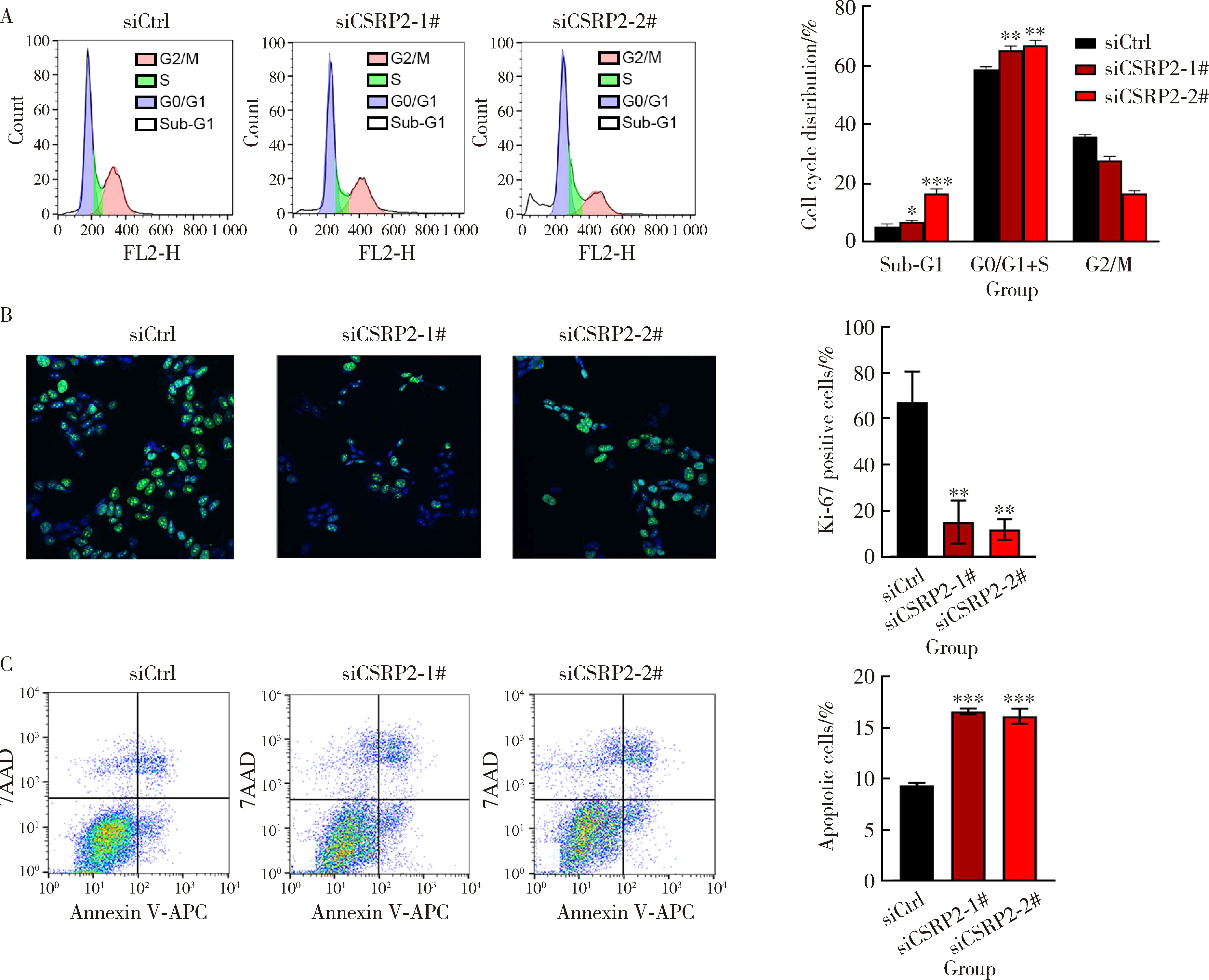
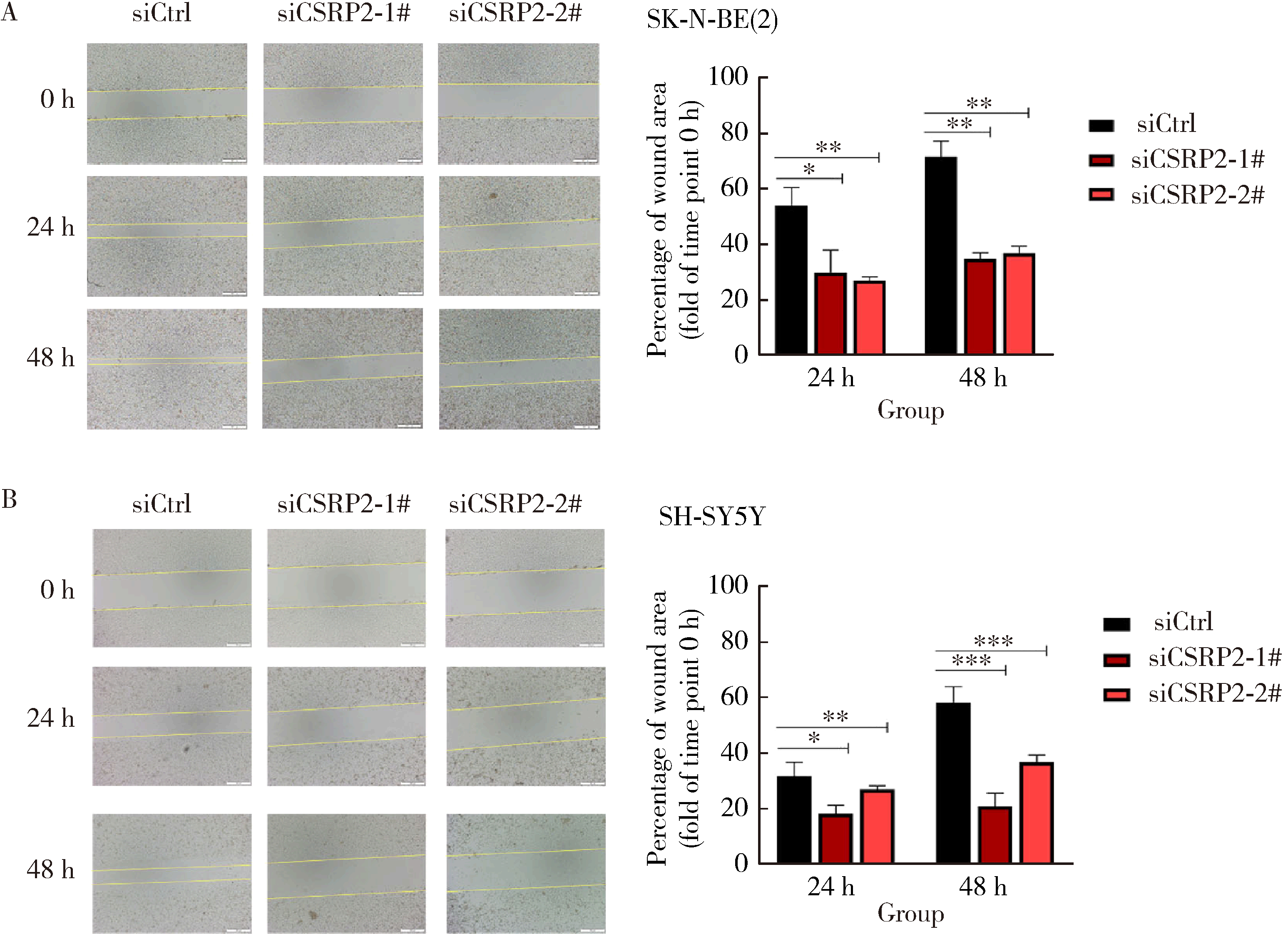
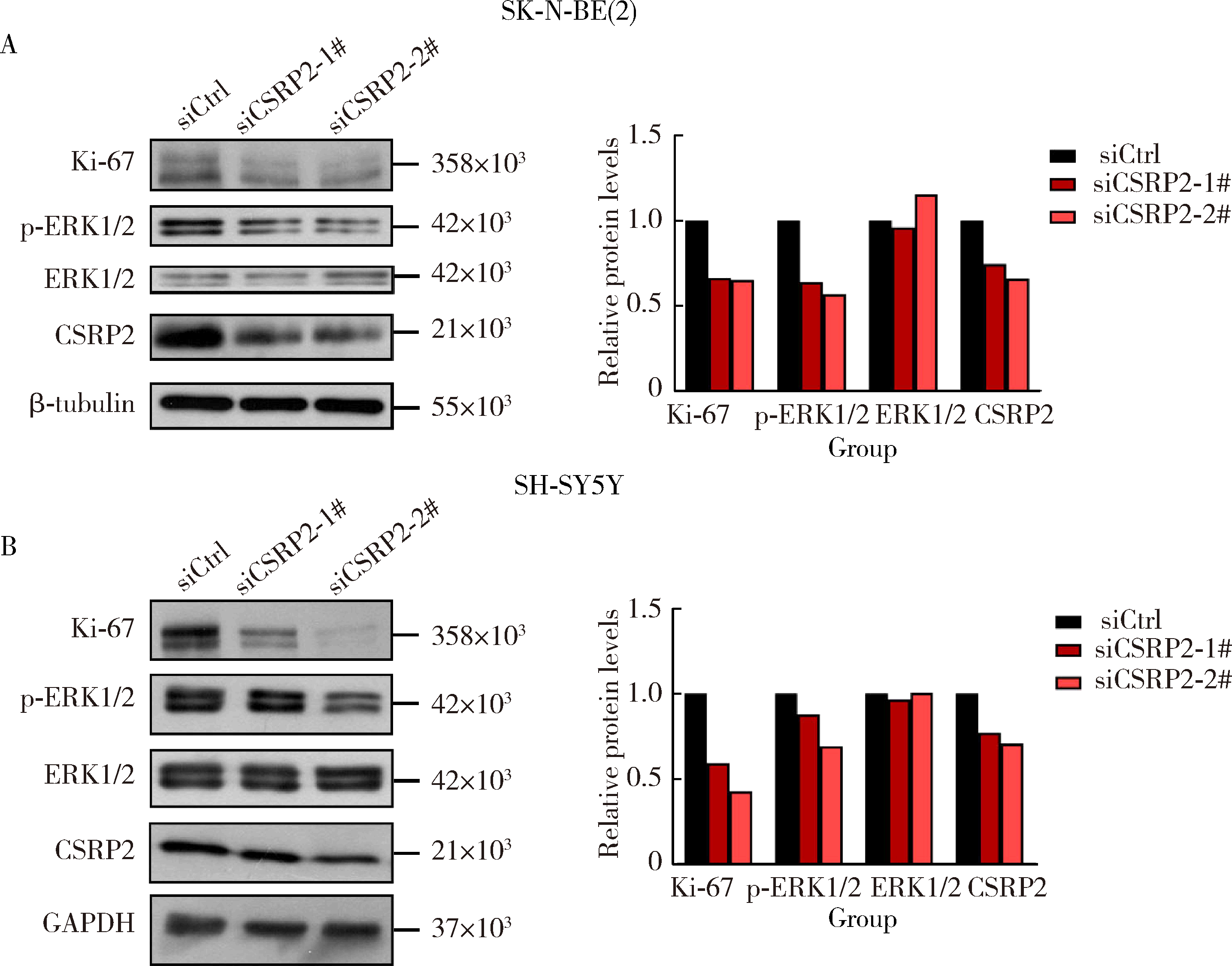
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (3): 495-504. doi: 10.19723/j.issn.1671-167X.2024.03.017
Previous Articles Next Articles
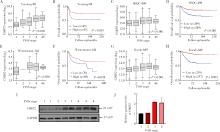
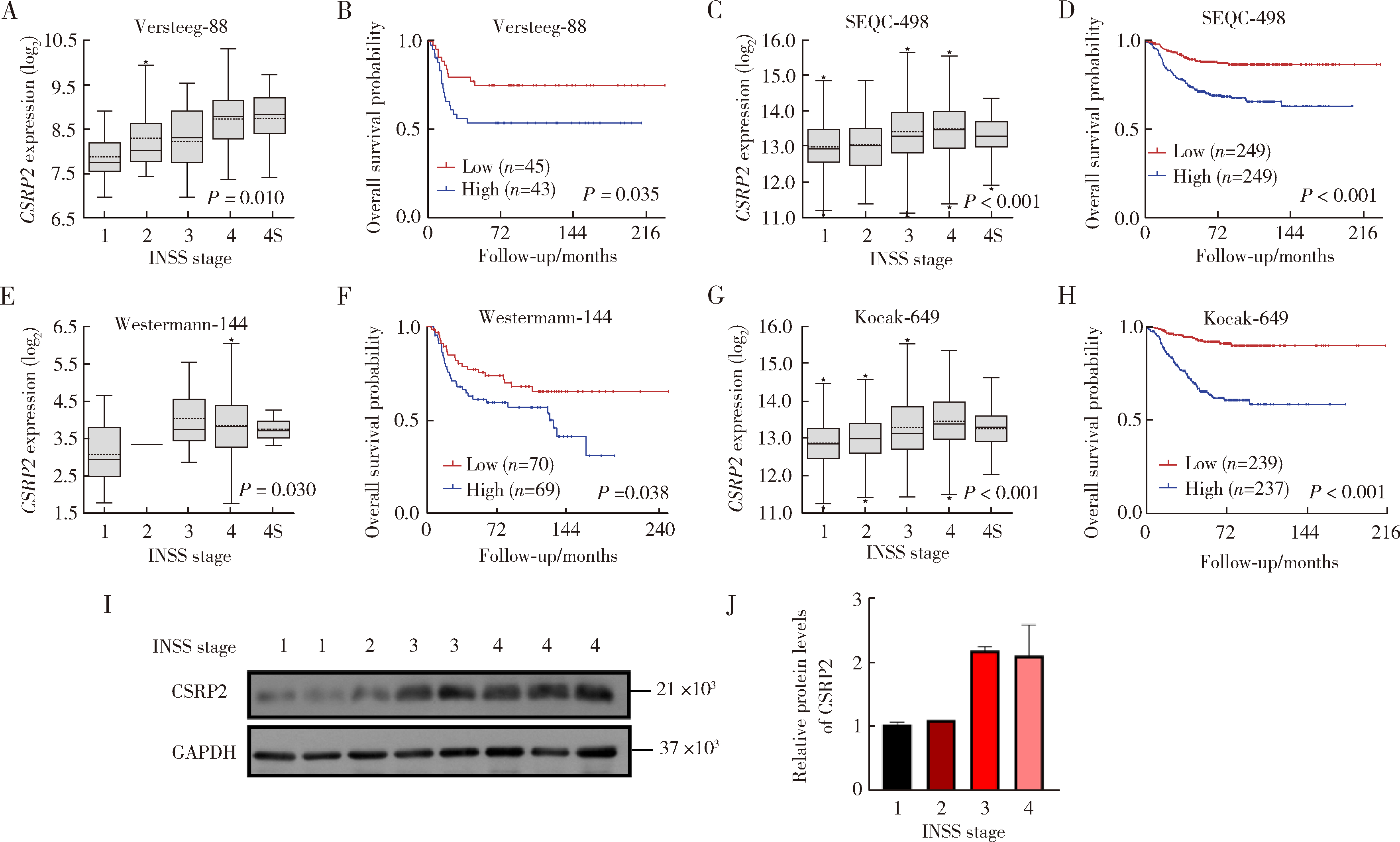
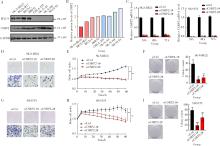
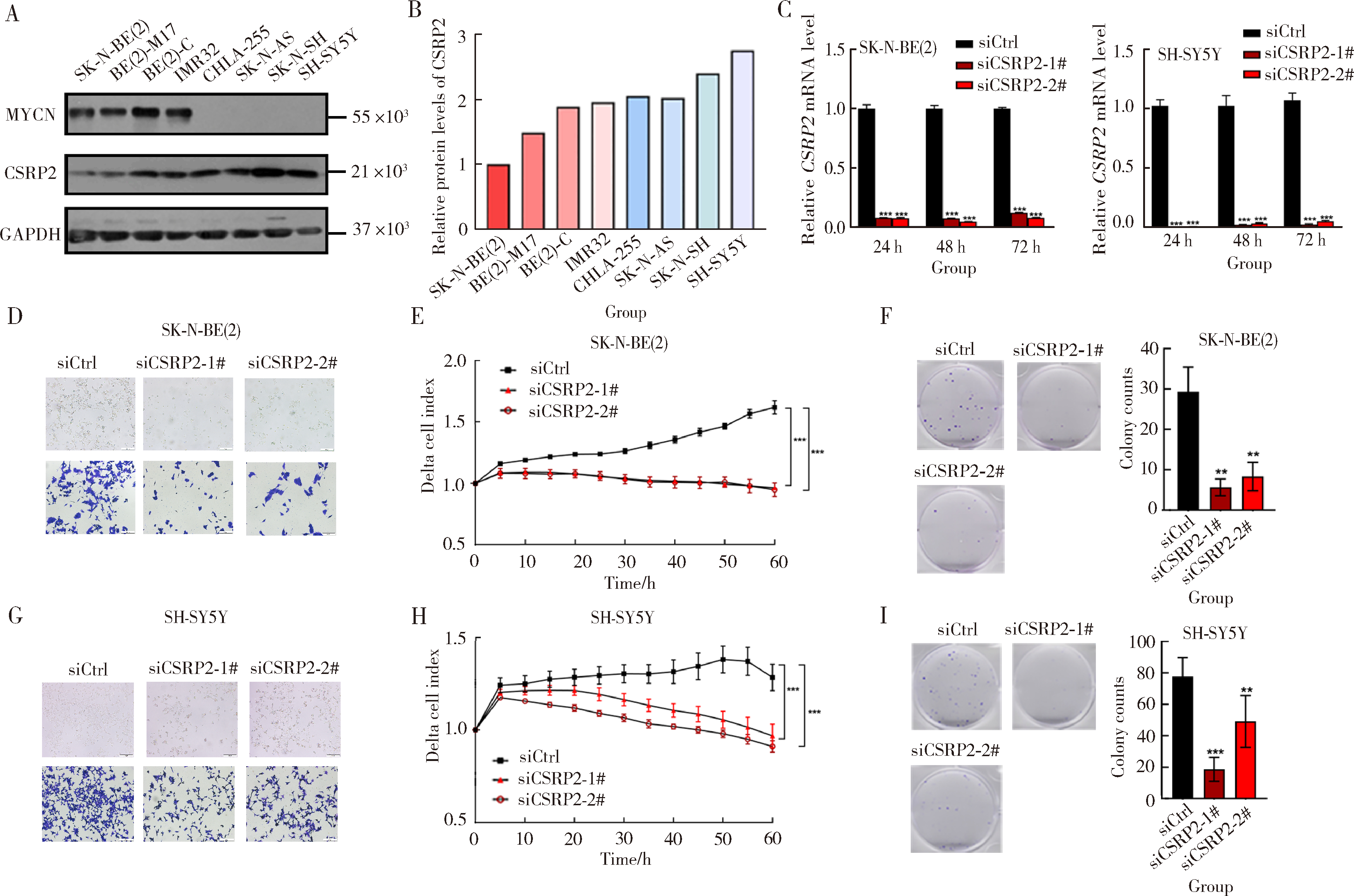
Role and mechanism of cysteine and glycine-rich protein 2 in the malignant progression of neuroblastoma
Yao ZHANG,Jinxin GUO,Shijia ZHAN,Enyu HONG,Hui YANG,Anna JIA,Yan CHANG,Yongli GUO,Xuan ZHANG*( )
)
- National Center for Children's Health; Beijing Children's Hospital, Capital Medical University; Key Laboratory of Major Diseases in Children, Ministry of Education; Beijing Pediatric Research Institute; Beijing Key Laboratory for Pediatric Diseases of Otolaryngology, Head and Neck Surgery; Beijing 100045, China
CLC Number:
- R739.4
| 1 |
Anderson J , Majzner RG , Sondel PM . Immunotherapy of neuroblastoma: Facts and hopes[J]. Clin Cancer Res, 2022, 28 (15): 3196- 3206.
doi: 10.1158/1078-0432.CCR-21-1356 |
| 2 | Zafar A , Wang W , Liu G , et al. Molecular targeting therapies for neuroblastoma: Progress and challenges[J]. Med Res Rev, 2020, 41 (2): 961- 1021. |
| 3 |
Su Y , Qin H , Chen C , et al. Treatment and outcomes of 1041 pediatric patients with neuroblastoma who received multidisciplinary care in China[J]. Pediatr Investig, 2020, 4 (3): 157- 167.
doi: 10.1002/ped4.12214 |
| 4 |
Sainero-Alcolado L , Mushtaq M , Liano-Pons J , et al. Expression and activation of nuclear hormone receptors result in neuronal differentiation and favorable prognosis in neuroblastoma[J]. J Exp Clin Cancer Res, 2022, 41 (1): 226.
doi: 10.1186/s13046-022-02399-x |
| 5 |
Maris JM . Recent advances in neuroblastoma[J]. N Engl J Med, 2010, 362 (23): 2202- 2211.
doi: 10.1056/NEJMra0804577 |
| 6 |
Jain MK , Kashiki S , Hsieh CM , et al. Embryonic expression suggests an important role for CRP2/SmLIM in the developing cardiovascular system[J]. Circ Res, 1998, 83 (10): 980- 985.
doi: 10.1161/01.RES.83.10.980 |
| 7 |
Sala S , Oakes PW . LIM domain proteins[J]. Curr Biol, 2023, 33 (9): R339- R341.
doi: 10.1016/j.cub.2023.03.030 |
| 8 |
Chen L , Long X , Duan S , et al. CSRP2 suppresses colorectal cancer progression via p130Cas/Rac1 axis-meditated ERK, PAK, and HIPPO signaling pathways[J]. Theranostics, 2020, 10 (24): 11063- 11079.
doi: 10.7150/thno.45674 |
| 9 |
Wang SJ , Wang PZ , Gale RP , et al. Cysteine and glycine-rich protein 2 (CSRP2) transcript levels correlate with leukemia relapse and leukemia-free survival in adults with B-cell acute lymphoblastic leukemia and normal cytogenetics[J]. Oncotarget, 2017, 8 (22): 35984- 36000.
doi: 10.18632/oncotarget.16416 |
| 10 |
Wang S , Zhang Y , Liu Y , et al. Inhibition of CSRP2 promotes leukemia cell proliferation and correlates with relapse in adults with acute myeloid leukemia[J]. Onco Targets Ther, 2020, 13, 12549- 12560.
doi: 10.2147/OTT.S281802 |
| 11 |
Hoffmann C , Mao X , Brown-Clay J , et al. Hypoxia promotes breast cancer cell invasion through HIF-1α-mediated up-regulation of the invadopodial actin bundling protein CSRP2[J]. Sci Rep, 2018, 8 (1): 10191.
doi: 10.1038/s41598-018-28637-x |
| 12 |
Bansal D , Totadri S , Chinnaswamy G , et al. Management of neuroblastoma: ICMR consensus document[J]. Indian J Pediatr, 2017, 84 (6): 446- 455.
doi: 10.1007/s12098-017-2298-0 |
| 13 | Guo YJ , Pan WW , Liu SB , et al. ERK/MAPK signalling pathway and tumorigenesis[J]. Exp Ther Med, 2020, 19 (3): 1997- 2007. |
| 14 |
Flynn SM , Lesperance J , Macias A , et al. The multikinase inhi-bitor RXDX-105 is effective against neuroblastoma in vitro and in vivo[J]. Oncotarget, 2019, 10 (59): 6323- 6333.
doi: 10.18632/oncotarget.27259 |
| 15 |
Janssen M , Schmidt C , Bruch PM , et al. Venetoclax synergizes with gilteritinib in FLT3 wild-type high-risk acute myeloid leukemia by suppressing MCL-1[J]. Blood, 2022, 140 (24): 2594- 2610.
doi: 10.1182/blood.2021014241 |
| 16 |
Chen L , Willis SN , Wei A , et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function[J]. Mol Cell, 2005, 17 (3): 393- 403.
doi: 10.1016/j.molcel.2004.12.030 |
| 17 | Mendoza MC , Vilela M , Juarez JE , et al. ERK reinforces actin polymerization to power persistent edge protrusion during motility[J]. Sci Signal, 2015, 8 (377): ra47. |
| 18 |
Hohmann T , Dehghani F . The cytoskeleton: A complex interacting meshwork[J]. Cells, 2019, 8 (4): 362.
doi: 10.3390/cells8040362 |
| 19 |
Hayashi KI , Horoiwa S , Mori K , et al. Role of CRP2-MRTF interaction in functions of myofibroblasts[J]. Cell Struct Funct, 2023, 48 (1): 83- 98.
doi: 10.1247/csf.23004 |
| 20 |
Chen CH , Ho HH , Jiang WC , et al. Cysteine-rich protein 2 deficiency attenuates angiotensin Ⅱ-induced abdominal aortic aneurysm formation in mice[J]. J Biomed Sci, 2022, 29 (1): 25.
doi: 10.1186/s12929-022-00808-z |
| 21 | Grubinger M , Gimona M . CRP2 is an autonomous actin-binding protein[J]. FEBS Lett, 2004, 557 (1/2/3): 88- 92. |
| 22 |
Moreno L , Barone G , Dubois SG , et al. Accelerating drug deve-lopment for neuroblastoma: Summary of the Second Neuroblastoma Drug Development Strategy forum from Innovative Therapies for Children with Cancer and International Society of Paediatric Onco-logy Europe Neuroblastoma[J]. Eur J Cancer, 2020, 136, 52- 68.
doi: 10.1016/j.ejca.2020.05.010 |
| 23 |
Wienke J , Dierselhuis MP , Tytgat GAM , et al. The immune landscape of neuroblastoma: Challenges and opportunities for novel therapeutic strategies in pediatric oncology[J]. Eur J Cancer, 2021, 144, 123- 150.
doi: 10.1016/j.ejca.2020.11.014 |
| 24 |
Wang L , Chen C , Song Z , et al. EZH2 depletion potentiates MYC degradation inhibiting neuroblastoma and small cell carcinoma tumor formation[J]. Nat Commun, 2022, 13 (1): 12.
doi: 10.1038/s41467-021-27609-6 |
| 25 | Chen L , Alexe G , Dharia NV , et al. CRISPR-Cas9 screen reveals a MYCN-amplified neuroblastoma dependency on EZH2[J]. J Clin Invest, 2018, 128 (1): 446- 462. |
| 26 |
Pacenta HL , Macy ME . Entrectinib and other ALK/TRK inhibitors for the treatment of neuroblastoma[J]. Drug Des Devel Ther, 2018, 12, 3549- 3561.
doi: 10.2147/DDDT.S147384 |
| 27 |
Temple WC , Vo KT , Matthay KK , et al. Association of image-defined risk factors with clinical features, histopathology, and outcomes in neuroblastoma[J]. Cancer Med, 2021, 10 (7): 2232- 2241.
doi: 10.1002/cam4.3663 |
| 28 |
Mina M , Boldrini R , Citti A , et al. Tumor-infiltrating T lymphocytes improve clinical outcome of therapy-resistant neuroblastoma[J]. Oncoimmunology, 2015, 4 (9): e1019981.
doi: 10.1080/2162402X.2015.1019981 |
| 29 |
Maerken TV , Speleman F , Vermeulen J , et al. Small-molecule MDM2 antagonists as a new therapy concept for neuroblastoma[J]. Cancer Res, 2006, 66 (19): 9646- 9655.
doi: 10.1158/0008-5472.CAN-06-0792 |
| 30 |
Xue C , Haber M , Flemming C , et al. p53 determines multidrug sensitivity of childhood neuroblastoma[J]. Cancer Res, 2007, 67 (21): 10351- 10360.
doi: 10.1158/0008-5472.CAN-06-4345 |
| 31 | Greengard EG . Molecularly targeted therapy for neuroblastoma[J]. Children (Basel), 2018, 5 (10): 142. |
| [1] | Jing XIE,Yu-ming ZHAO,Nan-quan RAO,Xiao-tong WANG,Teng-jiao-zi FANG,Xiao-xia LI,Yue ZHAI,Jing-zhi LI,Li-hong GE,Yuan-yuan WANG. Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 900-906. |
| [2] | TANG Xu, ZHAO Wei-hong, SONG Qin-qin, YIN Hua-qi, DU Yi-qing, SHENG Zheng-zuo, WANG Qiang, ZHANG Xiao-wei, LI Qing, LIU Shi-jun, XU Tao. Influence of SOX10 on the proliferation and invasion of prostate cancer cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 602-606. |
| [3] | WANG Zi-cheng, CHENG Li, LV Tong-de, SU Li, LIN Jian, ZHOU Li-qun. Inflammatory priming adipose derived stem cells significantly inhibit the proliferation of peripheral blood mononuclear cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 590-594. |
| [4] | CHEN Wei, HU Fan-lei, LIU Hong-jiang, XU Li-ling, LI Ying-ni, LI Zhan-guo. Myeloid-derived suppressor cells promoted autologous B cell proliferation in rheumatoid arthritis [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 819-823. |
| [5] | CAI Yi, GUO Hao, LI Han-zhong, WANG Wen-da, ZHANG Yu-shi. MicroRNA differential expression profile in tuberous sclerosis complex cell line TSC2-/- MEFs and normal cell line TSC2+/+ MEFs [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 580-584. |
| [6] | SIMA Zi-han, HONG Ying-ying, LI Tie-jun△. Effects of PTCH1 mutations on the epithelial proliferation derived from keratocystic odontogenic tumour [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 522-526. |
| [7] | GAO Xiang, CHEN Xiang-mei, ZHANG Ting, ZHANG Jing, CHEN Mo, GUO Zheng--yang, SHI Yan-yan, LU Feng-min, DING Shi-gang. Relationship between macrophage capping protein and gastric cancer cell’s proliferation and migration ability [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 489-494. |
| [8] | YANG Di, XU Jun-hui, DENG Fu-rong△, GUO Xin-biao . Effects of silver nanoparticle on hemichannel activation and anti-proliferation in HaCaT cells [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 371-375. |
| [9] | SUI Hua-xin, LV Pei-jun, WANG Yu-guang, WANG Yong, SUN Yu-chun. Effect of lowlevel laser irradiation on proliferation and osteogenic differentiation of human adipose-derived stromal cells [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 337-343. |
| [10] | LI Jing-wen, YIN Xiao-hui, LUAN Qing-xian. Comparative study of proliferative and periodontal differentiation propensity of induced pluripotent stem cells at different passages [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 16-024. |
| [11] | LING Long, ZHAO Yu-ming, GE Li-hong. Impact of different degree pulpitis on cell proliferation and osteoblastic differentiation of dental pulp stem cell in Beagle immature premolars [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 878-883. |
| [12] | HU Jia,ZOU Xiao-ying,ZHUANG Heng,GAO Xue-jun. Effect of root canal sealers on biocompatibility of human periodontal ligament cells [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 871-877. |
| [13] | HU Feng-zhan, YUAN Wan-qiong, WANG Xiao-lin, QIN Cai-peng, SHENG Zheng-zuo, DU Yi-qing, YIN Hua-qi, XU Tao. Knockdown of CMTM3 promotes migration and invasion of PC3 cell in vitro [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 594-597. |
| [14] | MO Heng, GAO Cheng-zhi, WANG Shao-jie, LI Mei, DONG Jian-qiang,YU Wei-dong . Expression, roles and therapy target values of CD24 in oral squamous cell carcinoma [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 16-22. |
| [15] | WANG Xiao-fei, LV Pei-jun, SONG Yang, WANG Yong, SUN Yu-chun. Short-term effect of CaCl2 on human adipose-derived mesenchymal stem cells proliferation and osteogenic differentiation [J]. Journal of Peking University(Health Sciences), 2015, 47(6): 971-976. |
|
||