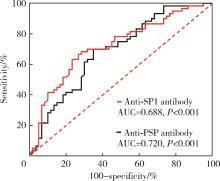
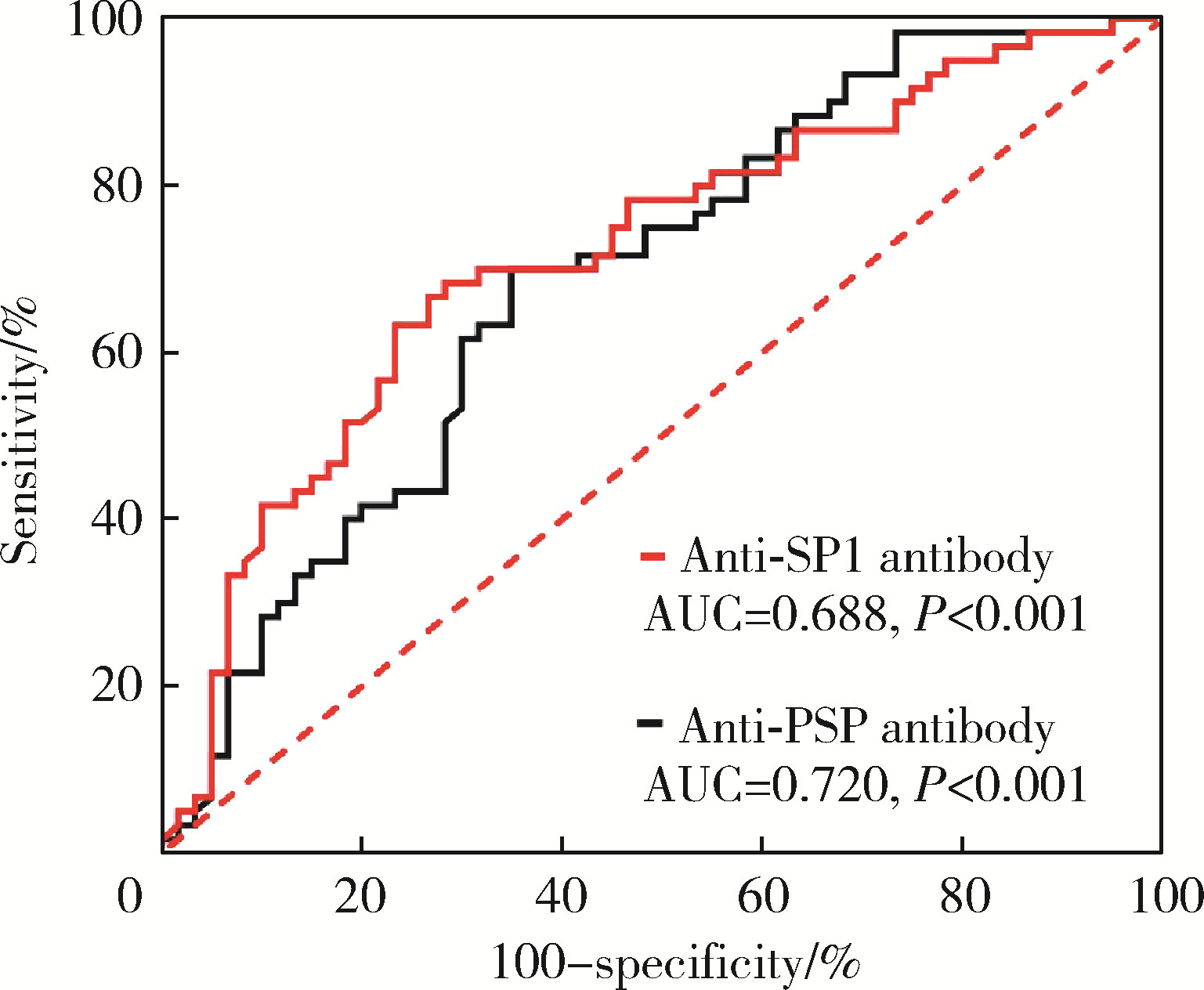
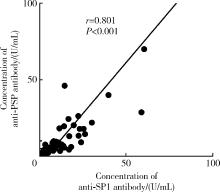
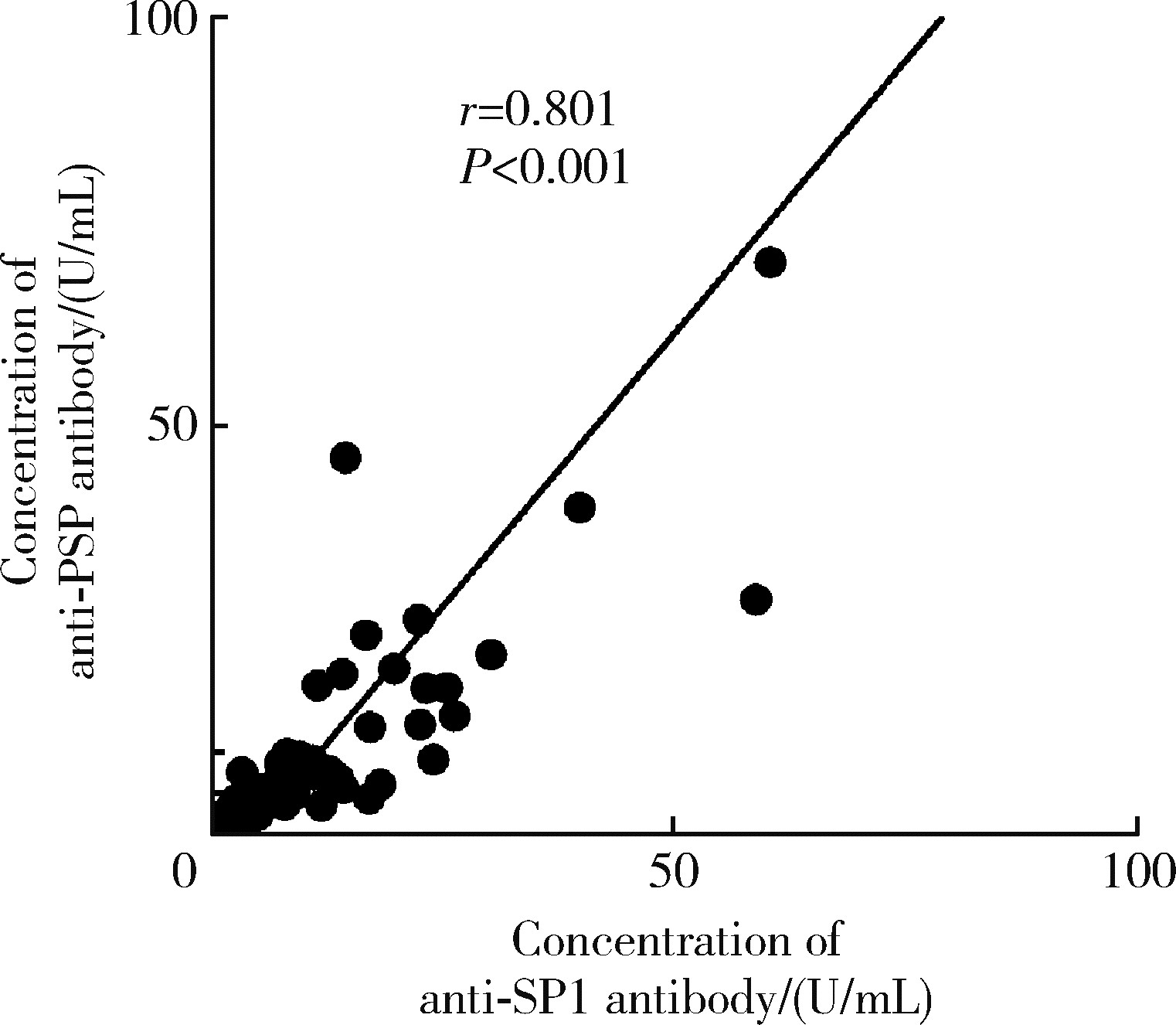
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (5): 845-852. doi: 10.19723/j.issn.1671-167X.2024.05.015
Previous Articles Next Articles
Diagnostic values of anti-salivary gland protein-1 antibody combined with anti-parotid secretory protein antibody for Sjögren's syndrome
Yushu YANG, Xuan QI, Meng DING, Wei WANG, Huifang GUO, Lixia GAO*( )
)
- Department of Rheumatology and Immunology, the Second Hospital of Hebei Medical University, Shijiazhuang 050011, China
CLC Number:
- R593.2
| 1 | Goules AV , Argyropoulou OD , Pezoulas VC , et al. Primary Sjögren's syndrome of early and late onset: Distinct clinical phenotypes and lymphoma development[J]. Front Immunol, 2020, 11, 594096. |
| 2 | Vitali C , Bombardieri S , Jonsson R , et al. Classification criteria for Sjögren's syndrome: A revised version of the European criteria proposed by the American-European Consensus Group[J]. Ann Rheum Dis, 2002, 61 (6): 554- 558. |
| 3 | Shiboski SC , Shiboski CH , Criswell L , et al. American College of Rheumatology classification criteria for Sjögren's syndrome: A data-driven, expert consensus approach in the Sjögren's International Collaborative Clinical Alliance cohort[J]. Arthritis Care Res (Hoboken), 2012, 64 (4): 475- 487. |
| 4 | Shiboski CH , Shiboski SC , Seror R , et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data-driven methodology involving three international patient cohorts[J]. Ann Rheum Dis, 2017, 76 (1): 9- 16. |
| 5 | Hoshina Y , Wong KH , Galli J , et al. Neurologic involvement in seronegative primary Sjögren's syndrome with positive minor salivary gland biopsy: A single-center experience[J]. Front Neurol, 2023, 14, 1174116. |
| 6 | Chen J , He Q , Yang J , et al. Anti-SSA/SSB-negative primary Sjögren's syndrome showing different clinical phenotypes: A retrospective study of 934 cases[J]. Adv Rheumatol, 2023, 63 (1): 21. |
| 7 | Bodeutsch C , de Wilde PC , Kater L , et al. Labial salivary gland biopsy in Sjögren's syndrome[J]. Neth J Med, 1992, 40 (3/4): 148- 157. |
| 8 | Theander E , Jonsson R , Sjöström B , et al. Prediction of Sjögren's syndrome years before diagnosis and identification of patients with early onset and severe disease course by autoantibody profiling[J]. Arthritis Rheumatol, 2015, 67 (9): 2427- 2436. |
| 9 | Jonsson R , Theander E , Sjöström B , et al. Autoantibodies present before symptom onset in primary Sjögren syndrome[J]. JAMA, 2013, 310 (17): 1854- 1855. |
| 10 | Lee AYS , Lin MW . Serological intermolecular epitope spreading in a patient with primary Sjögren's syndrome[J]. BMJ Case Rep, 2023, 16 (5): e254632. |
| 11 | Mona M , Mondello S , Hyon JY , et al. Clinical usefulness of anti-muscarinic type 3 receptor autoantibodies in patients with primary Sjögren's syndrome[J]. Clin Exp Rheumatol, 2021, 39 (4): 795- 803. |
| 12 | Alam J , Koh JH , Kwok SK , et al. Functional epitopes for anti-aquaporin 5 antibodies in Sjögren syndrome[J]. J Dent Res, 2017, 96 (12): 1414- 1421. |
| 13 | Alam J , Koh JH , Kim N , et al. Detection of autoantibodies against aquaporin-5 in the sera of patients with primary Sjögren's syndrome[J]. Immunol Res, 2016, 64 (4): 848- 856. |
| 14 | Tjensvoll AB , Lauvsnes MB , Zetterberg H , et al. Neurofilament light is a biomarker of brain involvement in lupus and primary Sjögren's syndrome[J]. Journal of Neurology, 2021, 268 (4): 1385- 1394. |
| 15 | Shen L , Gao C , Suresh L , et al. Central role for marginal zone B cells in an animal model of Sjögren's syndrome[J]. Clin Immunol, 2016, 168, 30- 36. |
| 16 | Jin Y , Li J , Chen J , et al. Tissue-specific autoantibodies improve diagnosis of primary Sjögren's syndrome in the early stage and indicate localized salivary injury[J]. J Immunol Res, 2019, 2019, 3642937. |
| 17 | Shen L , Suresh L , Lindemann M , et al. Novel autoantibodies in Sjögren's syndrome[J]. Clin Immunol, 2012, 145 (3): 251- 255. |
| 18 | Xuan J , Wang Y , Xiong Y , et al. Investigation of autoantibodies to SP-1 in chinese patients with primary Sjögren's syndrome[J]. Clin Immunol, 2018, 188, 58- 63. |
| 19 | Xian Z , Fu D , Liu S , et al. Association between B cell growth factors and primary Sjögren's syndrome-related autoantibodies in patients with non-Hodgkin's lymphoma[J]. J Immunol Res, 2019, 2019, 7627384. |
| 20 | Bunya VY , Massaro-Giordano M , Vivino FB , et al. Prevalence of novel candidate Sjögren syndrome autoantibodies in the penn Sjögren's International Collaborative Clinical Alliance Cohort[J]. Cornea, 2019, 38 (12): 1500- 1505. |
| 21 | Shen L , Kapsogeorgou EK , Yu M , et al. Evaluation of salivary gland protein 1 antibodies in patients with primary and secondary Sjögren's syndrome[J]. Clin Immunol, 2014, 155 (1): 42- 46. |
| 22 | Applbaum E , Lichtbroun A . Novel Sjögren's autoantibodies found in fibromyalgia patients with sicca and/or xerostomia[J]. Autoimmun Rev, 2019, 18 (2): 199- 202. |
| 23 | de Langhe E , Bossuyt X , Shen L , et al. Evaluation of autoantibodies in patients with primary and secondary Sjögren's syndrome[J]. Open Rheumatol J, 2017, 11, 10- 15. |
| 24 | Hubschman S , Rojas M , Kalavar M , et al. Association between early Sjögren markers and symptoms and signs of dry eye[J]. Cornea, 2020, 39 (3): 311- 315. |
| 25 | Geetha C , Venkatesh SG , Dunn BH , et al. Expression and anti-bacterial activity of human parotid secretory protein (PSP)[J]. Biochem Soc Trans, 2003, 31 (Pt 4): 815- 818. |
| 26 | Karakus S , Baer AN , Akpek EK . Clinical correlations of novel autoantibodies in patients with dry eye[J]. J Immunol Res, 2019, 2019, 7935451. |
| 27 | Burbelo PD , Ferré E , Chaturvedi A , et al. Profiling autoanti-bodies against salivary proteins in sicca conditions[J]. J Dent Res, 2019, 98 (7): 772- 778. |
| 28 | Thatayatikom A , Jun I , Bhattacharyya I , et al. The diagnostic performance of early Sjögren's syndrome autoantibodies in juvenile Sjögren's syndrome: The university of florida pediatric cohort study[J]. Front Immunol, 2021, 12, 704193. |
| 29 | Lee KA , Kim KW , Kim BM , et al. Clinical and diagnostic signi-ficance of serum immunoglobulin A rheumatoid factor in primary Sjögren's syndrome[J]. Clin Oral Investig, 2019, 23 (3): 1415- 1423. |
| 30 | Zhong H , Wang Y , Yang P , et al. Hyperglobulinemia predicts increased risk of mortality in primary Sjögren's syndrome: Based on a Chinese multicentre registry[J]. Mod Rheumatol, 2023, 34 (1): 137- 143. |
| 31 | Kota SK , Pernicone E , Leaf DE , et al. BPI fold-containing family a member 2/parotid secretory protein is an early biomarker of AKI[J]. J Am Soc Nephrol, 2017, 28 (12): 3473- 3478. |
| [1] | Yijun HAN, Xiaoli CHEN, Changhong LI, Jinxia ZHAO. Application status of methotrexate in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 994-1000. |
| [2] | Jiayu ZHAI, Jinxia ZHAO, Zhuo AN, Rui LIU. Assessment of residual symptoms in patients with axial spondyloarthritis with low disease activity and analysis of its related factors [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 987-993. |
| [3] | Yujing ZHU, Lei WANG, Chengyin LYU, Wenfeng TAN, Miaojia ZHANG. Analysis of clinical features of ruccrent interstitial lung disease in patients with anti-EJ positive antisynthetase syndrome [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 980-986. |
| [4] | Liang ZHAO, Chenglong SHI, Ke MA, Jing ZHAO, Xiao WANG, Xiaoyan XING, Wanxing MO, Yirui LIAN, Chao GAO, Yuhui LI. Immunological characteristics of patients with anti-synthetase syndrome overlap with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 972-979. |
| [5] | Meijuan LONG, Yidan WANG, Shiya WU, Zihao LI, Yanting LI, Yang LI, Juan JIAO. Effects of overweight and obesity on symptoms, overall condition and quality of life in patients with fibromyalgia syndrome [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1001-1008. |
| [6] | Peiwen JIA, Ying YANG, Yaowei ZOU, Zhiming OUYANG, Jianzi LIN, Jianda MA, Kuimin YANG, Lie DAI. Clinical characteristics of overlapping syndromes of low muscle mass in patients with rheumatoid arthritis and their impact on physical function [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1009-1016. |
| [7] | Hongyan WANG, Xinming LI, Kechi FANG, Huaqun ZHU, Rulin JIA, Jing WANG. Analysis of characteristics related to the disease activity of systemic lupus erythematosus and construction of an evaluation model [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1017-1022. |
| [8] | Dandan CHEN, Yun LI, Qingyi LU, Xiaohong XIANG, Feng SUN, Yingni LI, Jing ZHAO, Hongyan WANG, Chun LI. Ovarian function in patients of childbearing age with systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1023-1028. |
| [9] | Li WANG, Chao GAO, Huanhuan REN, Yanping SHEN, Xiaowei HUANG, Hong YAO, Dandan HAN. Current status and influential factors of self-management ability in patients with systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1029-1035. |
| [10] | Yukai LI, Hongyan WANG, Liang LUO, Yun LI, Chun LI. Clinical significance of antiphospholipid antibodies in Behcet disease with thrombosis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1036-1040. |
| [11] | Wei PAN, Yun LI, Junjia LUO, Chun LI, Hua YE, Xue LI, Yuan JIA. COVID-19 vaccines efficacy and infection features in patients with systemic sclerosis: A single-center cohort study [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1041-1046. |
| [12] | Yan DING, Chaoran LI, Wensheng HUANG, Linzhong ZHU, Lifang WANG, Doudou MA, Juan ZHANG, Lianjie SHI. IgA vasculitis with necrosis of the small intestine secondary to monoclonal gammopathy of renal significance: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1101-1105. |
| [13] | Doudou MA, Zhemin LU, Qian GUO, Sha ZHU, Jin GU, Yan DING, Lianjie SHI. Successful treatment of rheumatoid arthritis complicated with myasthenia gravis with low-dose rituximab: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1110-1114. |
| [14] | Jing CHAI, Yue WANG, Rong MU, Jinxia ZHAO. Systemic lupus erythematosus involving the fornix column leading to hyponatremia: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1115-1118. |
| [15] | Mingxia WANG, Ling DING, Min WANG, Chanjuan ZOU, Siyu YAN, Yingwen LIANG, Weijia WANG, Shanzhi HE. Safe pregnancy and delivery in a female patient with systemic lupus erythematosus after discontinuation of dual-target chimeric antigen receptor T cells therapy [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1119-1125. |
|
||