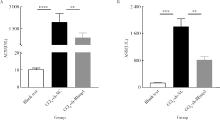
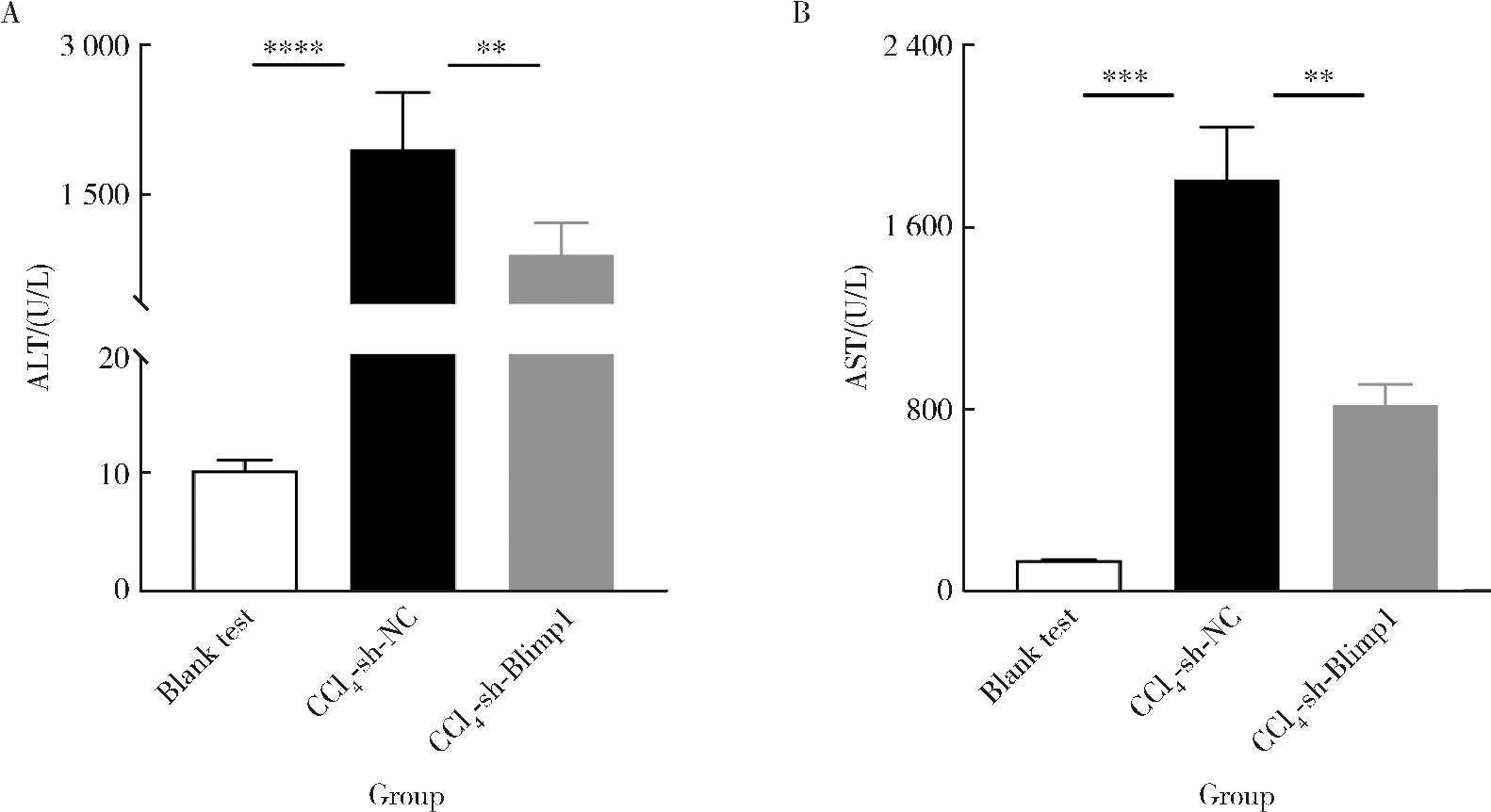
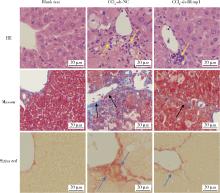
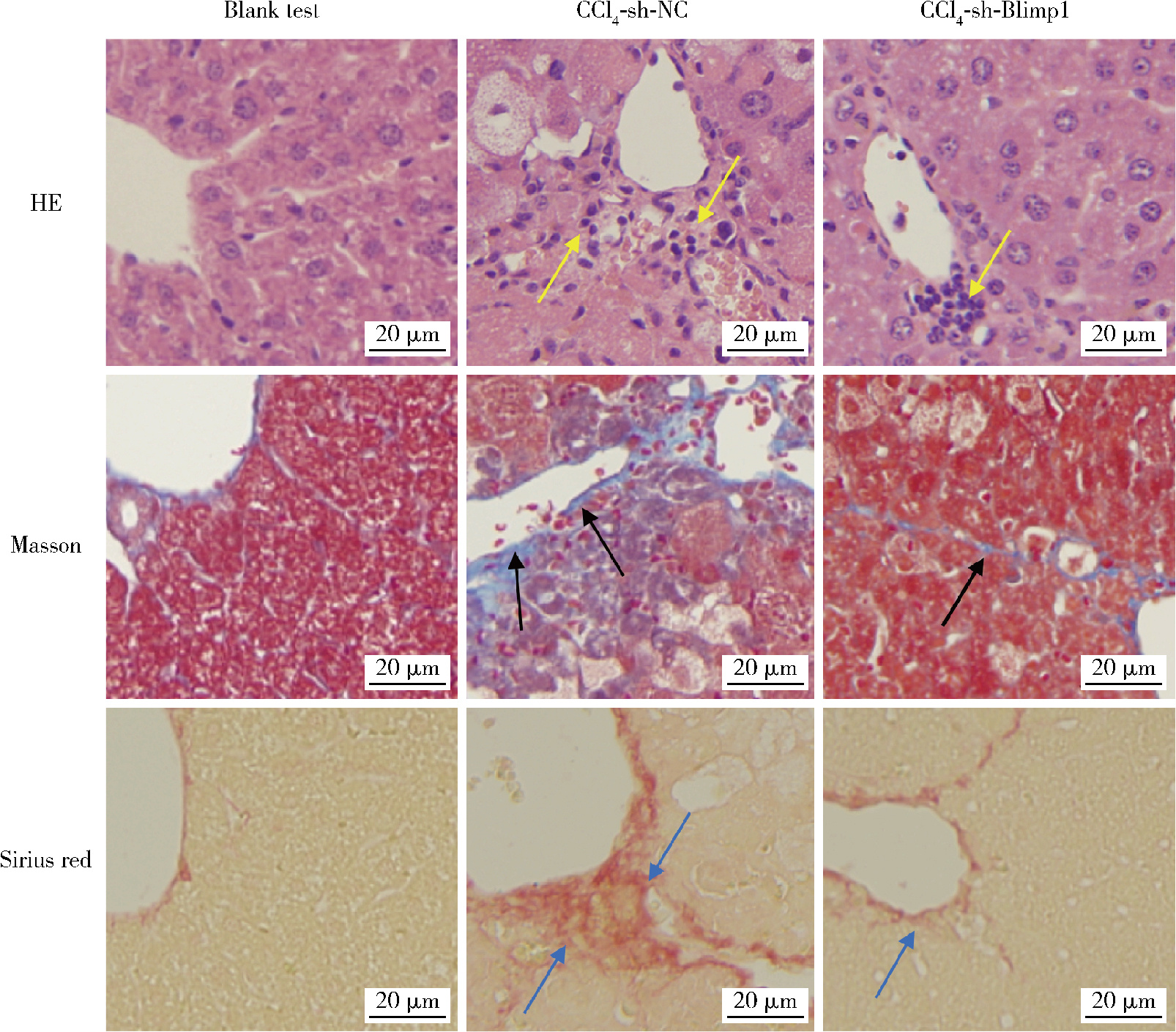
| 1 |
Marcellin P , Kutala BK . Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening[J]. Liver Int, 2018, 38 (Suppl 1): 2- 6.
|
| 2 |
Ramachandran P , Dobie R , Wilson-Kanamori JR , et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level[J]. Nature, 2019, 575 (7783): 512- 518.
|
| 3 |
Friedman SL , Pinzani M . Hepatic fibrosis 2022:Unmet needs and a blueprint for the future[J]. Hepatology, 2022, 75 (2): 473- 488.
|
| 4 |
Rockey DC , Bell PD , Hill JA . Fibrosis: A common pathway to organ injury and failure[J]. N Engl J Med, 2015, 372 (12): 1138- 1149.
|
| 5 |
Shapiro-Shelef M , Lin KI , McHeyzer-Williams LJ , et al. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells[J]. Immunity, 2003, 19 (4): 607- 620.
|
| 6 |
Minnich M , Tagoh H , Bönelt P , et al. Multifunctional role of the transcription factor Blimp-1 in coordinating plasma cell differentiation[J]. Nat Immunol, 2016, 17 (3): 331- 343.
|
| 7 |
Bankoti R , Ogawa C , Nguyen T , et al. Differential regulation of effector and regulatory T cell function by Blimp1[J]. Sci Rep, 2017, 7 (1): 12078.
|
| 8 |
Shin H , Blackburn SD , Intlekofer AM , et al. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection[J]. Immunity, 2009, 31 (2): 309- 320.
|
| 9 |
Savitsky D , Cimmino L , Kuo T , et al. Multiple roles for Blimp-1 in B and T lymphocytes[J]. Adv Exp Med Biol, 2007, 596, 9- 30.
|
| 10 |
Kim SJ , Zou YR , Goldstein J , et al. Tolerogenic function of Blimp-1 in dendritic cells[J]. J Exp Med, 2011, 208 (11): 2193- 2199.
|
| 11 |
Gateva V , Sandling JK , Hom G , et al. A large-scale replication study identifies TNIP1, PRDM1, JAZF1, UHRF1BP1 and IL10 as risk loci for systemic lupus erythematosus[J]. Nat Genet, 2009, 41 (11): 1228- 1233.
|
| 12 |
Ellinghaus D , Zhang H , Zeissig S , et al. Association between variants of PRDM1 and NDP52 and Crohn ' s disease, based on exome sequencing and functional studies[J]. Gastroenterology, 2013, 145 (2): 339- 347.
|
| 13 |
Boi M , Zucca E , Inghirami G , et al. PRDM1/BLIMP1:A tumor suppressor gene in B and T cell lymphomas[J]. Leuk Lymphoma, 2015, 56 (5): 1223- 1228.
|
| 14 |
Li N , Fan X , Wang X , et al. PRDM1 levels are associated with clinical diseases in chronic HBV infection and survival of patients with HBV-related hepatocellular carcinoma[J]. Int Immunopharmacol, 2019, 73, 156- 162.
|
| 15 |
Scholten D , Trebicka J , Liedtke C , et al. The carbon tetrachloride model in mice[J]. Lab Anim, 2015, 49 (Suppl 1): 4- 11.
|
| 16 |
Bárcena C , Aran G , Perea L , et al. CD5L is a pleiotropic player in liver fibrosis controlling damage, fibrosis and immune cell content[J]. EBioMedicine, 2019, 43, 513- 524.
|
| 17 |
Pellicano AJ , Spahn K , Zhou P , et al. Collagen characterization in a model of nonalcoholic steatohepatitis with fibrosis: A call for development of targeted therapeutics[J]. Molecules, 2021, 26 (11): 3316.
|
| 18 |
Fan W , Liu T , Chen W , et al. ECM1 prevents activation of transforming growth factor β, hepatic stellate cells, and fibrogenesis in mice[J]. Gastroenterology, 2019, 157 (5): 1352- 1367.
|
| 19 |
Friedman SL . Hepatic stellate cells: Protean, multifunctional, and enigmatic cells of the liver[J]. Physiol Rev, 2008, 88 (1): 125- 172.
|
| 20 |
Xu XY , Ding HG , Li WG , et al. Chinese guidelines on management of hepatic encephalopathy in cirrhosis[J]. World J Gastroenterol, 2019, 25 (36): 5403- 5422.
|
| 21 |
Cao Y , Mai W , Li R , et al. Macrophages evoke autophagy of hepatic stellate cells to promote liver fibrosis in NAFLD mice via the PGE2/EP4 pathway[J]. Cell Mol Life Sci, 2022, 79 (6): 303.
|
| 22 |
Kiziltas S . Toll-like receptors in pathophysiology of liver diseases[J]. World J Hepatol, 2016, 8 (32): 1354- 1369.
|
| 23 |
Seki E , De Minicis S , Osterreicher CH , et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis[J]. Nat Med, 2007, 13 (11): 1324- 1332.
|
| 24 |
Genestier L , Taillardet M , Mondiere P , et al. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses[J]. J Immunol, 2007, 178 (12): 7779- 7786.
|
| 25 |
Ko YA , Chan YH , Liu CH , et al. Blimp-1-mediated pathway promotes type Ⅰ IFN production in plasmacytoid dendritic cells by targeting to interleukin-1 receptor-associated kinase M[J]. Front Immunol, 2018, 9, 1828.
|
| 26 |
Dewidar B , Meyer C , Dooley S , et al. TGF-β in hepatic stellate cell activation and liver fibrogenesis-updated 2019[J]. Cells, 2019, 8 (11): 1419.
|
| 27 |
Flores-Contreras L , Sandoval-Rodríguez AS , Mena-Enriquez MG , et al. Treatment with pirfenidone for two years decreases fibrosis, cytokine levels and enhances CB2 gene expression in patients with chronic hepatitis C[J]. BMC Gastroenterol, 2014, 14, 131.
|
 )
)