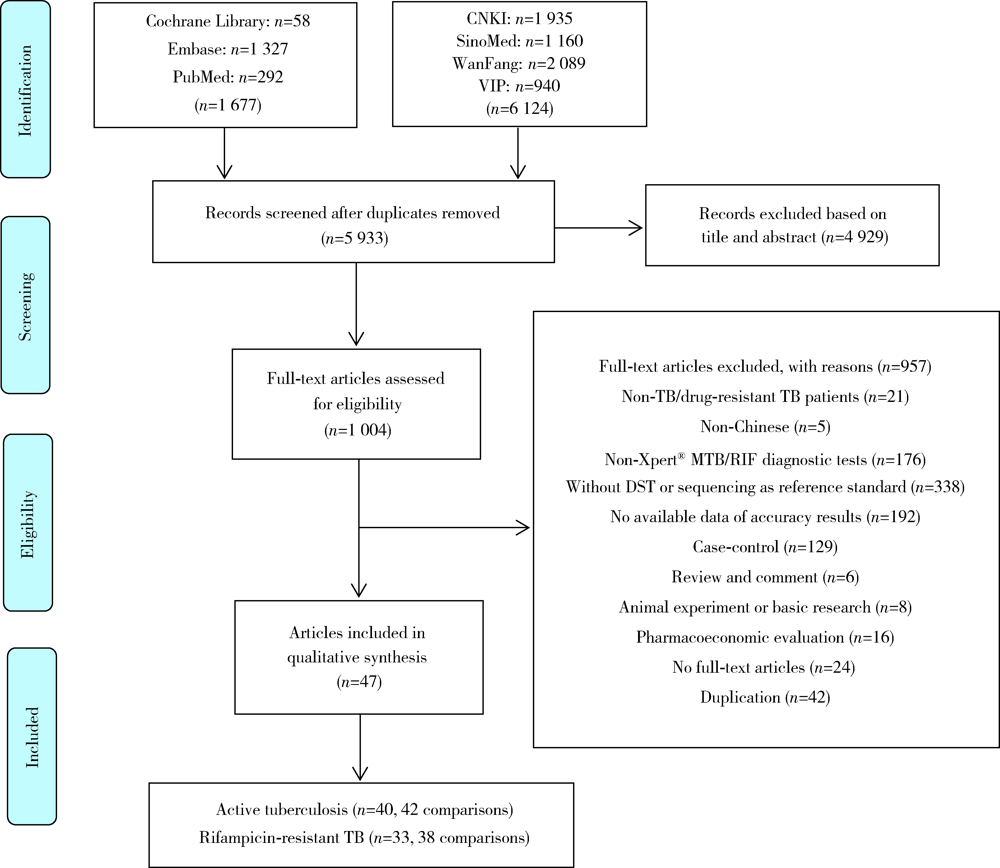
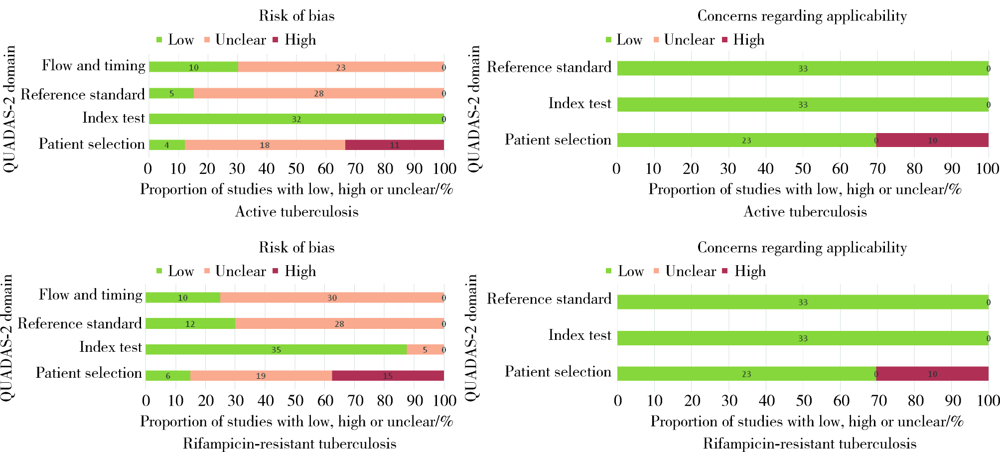
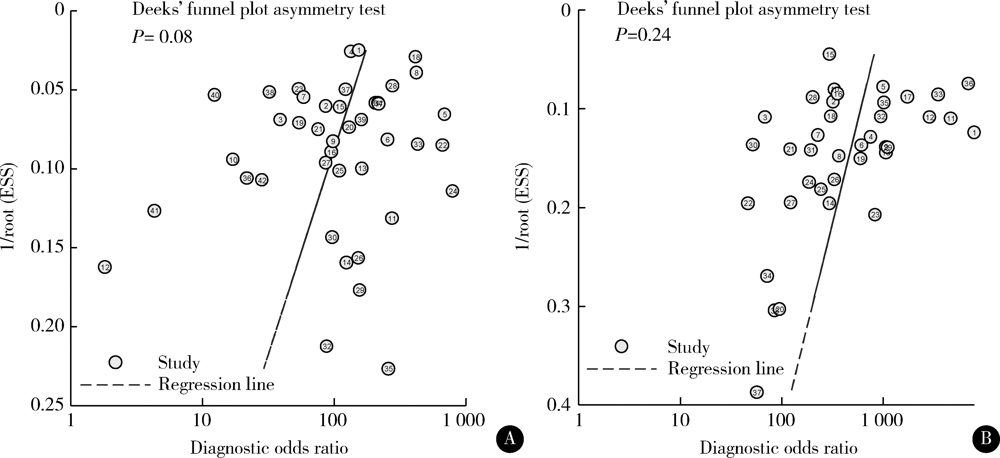
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (2): 320-326. doi: 10.19723/j.issn.1671-167X.2021.02.015
Previous Articles Next Articles
Accuracy of Xpert®MTB/RIF for the detection of tuberculosis and rifampicin-resistance tuberculosis in China: A systematic review and meta-analysis
FENG Jing-nan,GAO Le,SUN Yi-xin,YANG Ji-chun,DENG Si-wei,SUN Feng,ZHAN Si-yan( )
)
- Department of Epidemiology and Biostatistics, Peking University School of Public Health, Beijing 100191, China
CLC Number:
- R181.2
| [1] | WHO. Global tuberculosis report 2017 [EB/OL]. [2019-01-07]. http://www.who.int/tb/publications/global_report/en/. |
| [2] | WHO. The End TB Strategy [EB/OL]. [2018-12-16]. http://www.who.int/tb/strategy/en/. |
| [3] | 陆震宇, 杨明芳, 陈纬. 结核病实验室诊断技术评价及其研究进展[J]. 中国卫生检验杂志, 2017,27(2):301-304. |
| [4] | 刘宇, 吴利先. Xpert MTB/RIF在结核病诊断中的研究进展[J]. 中国病原生物学杂志, 2017,12(8):800-802. |
| [5] |
Ding P, Li X, Jia Z, et al. Multidrug-resistant tuberculosis (MDR-TB) disease burden in China: a systematic review and spatio-temporal analysis[J]. BMC Infect Dis, 2017,17(1):57.
doi: 10.1186/s12879-016-2151-5 pmid: 28073344 |
| [6] | PROSPERO. International prospective register of systematic reviews[EB/OL].[2018-12-11]. https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=93417. |
| [7] |
Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies[J]. Ann Intern Med, 2011,155(8):529-536.
pmid: 22007046 |
| [8] |
Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed[J]. J Clin Epidemiol, 2005,58(9):882-893.
doi: 10.1016/j.jclinepi.2005.01.016 pmid: 16085191 |
| [9] |
Liu Z, Pan A, Wu B, et al. Feasibility of a new model for early detection of patients with multidrug-resistant tuberculosis in a developed setting of eastern China[J]. Trop Med Int Health, 2017,22(10):1328-1333.
doi: 10.1111/tmi.12934 pmid: 28746979 |
| [10] | Ou X, Xia H, Li Q, et al. A feasibility study of the Xpert MTB/RIF test at the peripheral level laboratory in China[J]. Int J Infect Dis, 2015(31):41-46. |
| [11] |
Pang Y, Wang Y, Zhao S, et al. Evaluation of the Xpert MTB/RIF assay in gastric lavage aspirates for diagnosis of smear-negative childhood pulmonary tuberculosis[J]. Pediatr Infect Dis J, 2014,33(10):1047-1051.
doi: 10.1097/INF.0000000000000403 |
| [12] |
Shao Y, Peng H, Chen C, et al. Evaluation of GeneXpert MTB/RIF for detection of pulmonary tuberculosis at peripheral tuberculosis clinics[J]. Microb Pathog, 2017,105:260-263.
doi: 10.1016/j.micpath.2017.02.040 pmid: 28258004 |
| [13] |
Tang T, Liu F, Lu X, et al. Evaluation of GeneXpert MTB/RIF for detecting Mycobacterium tuberculosis in a hospital in China[J]. J Int Med Res, 2017,45(2):816-822.
doi: 10.1177/0300060517698618 pmid: 28351283 |
| [14] | Wang SF, Ou XC, Li Q, et al. The Abbott RealTime MTB assay and the Cepheid GeneXpert assay show comparable performance for the detection of Mycobacterium tuberculosis in sputum specimens[J]. Int J Infect Dis, 2016(45):78-80. |
| [15] | 曾松芳, 郭美丽, 赵珊珊, 等. 结核分枝杆菌/利福平耐药实时荧光定量核酸扩增检测技术在肺结核快速诊断中的应用价值[J]. 中国卫生检验杂志, 2016,26(5):683-685. |
| [16] | 陈玮, 李杨, 陈依江, 等. Xpert Mtb/RIF检测技术在县级肺结核诊断中的应用评价[J]. 中国卫生产业, 2017,14(17):5-7. |
| [17] | 陈子芳, 孙本海, 魏海冬, 等. GeneXpert MTB/RIF技术对菌阴肺结核患者辅助诊断的价值[J]. 中国防痨杂志, 2016,38(10):827-831. |
| [18] | 杜安玲. Xpert MTB/RIF技术检测肺结核病人痰菌阳性结果分析[J]. 河南预防医学杂志, 2017,28(3):204-205. |
| [19] | 高春景, 朱述阳. 支气管肺泡灌洗液行Xpert MTB/RIF检测对涂阴肺结核的诊断价值[J]. 临床肺科杂志, 2016,21(12):2192-2196. |
| [20] | 郭炽星, 蒋敏慧, 黎敬忠, 等. Xpert MTB/RIF系统检测痰标本的应用评价[J]. 广东医学院学报, 2016,34(1):67-69. |
| [21] | 郭婧玮, 袁仕善, 谭云洪, 等. Xpert MTB/RIF对肺结核患者痰标本中结核分枝杆菌及其利福平耐药快速检测的应用性研究[C]// 科学研究与结核病防治高峰论坛论文汇编. 陕西延安: 科学研究与结核病防治高峰论坛, 2014. |
| [22] | 韩丹, 段慧楠, 饶有益, 等. 纤维支气管镜灌洗液Xpert MTB/RIF检测对肺结核诊断和利福平耐药菌株筛选的临床价值[J]. 医学综述, 2016,22(16):3243-3246. |
| [23] | 何贵清, 李涛, 施伎蝉, 等. 利福平耐药结核分枝杆菌实时荧光定量核酸扩增检测技术在214例诊断肺结核患者中的临床应用评价[J]. 中华传染病杂志, 2016,34(6):349-353. |
| [24] | 黄芳, 党丽云, 孙惠平, 等. 三种分子生物学诊断技术对结核病诊断价值的比较[J]. 中华结核和呼吸杂志, 2015,38(9):680-685. |
| [25] | 黄建斌, 杨玲, 梁晓莉. GeneXpert MTB/RIF检测技术在结核病诊断中的应用探讨[J]. 医学动物防制, 2017,33(4):465-467. |
| [26] | 赖聪娟, 雷永良, 季柏林, 等. Xpert MTB/RIF技术辅助诊断耐多药肺结核的价值分析[J]. 中国卫生检验杂志, 2016,26(18):2628-2630. |
| [27] | 李静, 林日文, 张灿强. Xpert MTB/RIF检测痰标本结核分枝杆菌与利福平耐受性的临床应用研究[J]. 国际检验医学杂志, 2017,38(4):480-482. |
| [28] | 李妍, 张天华, 鲜小萍, 等. Xpert MTB/RIF检测技术诊断肺结核和肺外结核的价值[J]. 中国防痨杂志, 2015,37(6):586-589. |
| [29] | 林日文, 李静. 利福平耐药实时荧光定量核酸扩增技术检测333例肺结核患者痰标本的结果分析[J]. 国际检验医学杂志, 2016,37(20):2901-2902. |
| [30] | 林雪峰, 支晓阳. GeneXpert MTB/RIF实时荧光定量PCR系统在肺结核诊断及利福平耐药检测中的应用[J]. 中国卫生检验杂志, 2017,27(6):853-856. |
| [31] | 刘爱梅, 苏玉, 张琪, 等. GeneXpert MTB/RIF试验在肺结核诊断中的应用评价[J]. 中国医师进修杂志, 2017,40(6):500-504. |
| [32] | 刘畅. GeneXpert检测在肺结核病的临床诊断及治疗中的应用价值分析[J]. 医学理论与实践, 2016,29(24):3392-3394. |
| [33] | 刘建侠. Xpert MTB/RIF技术在基层定点医院肺结核病诊断中的应用价值[J]. 中国实验诊断学, 2016,20(6):928-930. |
| [34] | 刘涛, 杨翌翔, 刘军, 等. Xpert MTB/RIF检测技术诊断结核病及其耐药的研究[J]. 检验医学与临床, 2017,14(13):1898-1900. |
| [35] | 刘亚芹, 杨振斌, 冯冬霞, 等. GeneXpert法检测结核分枝杆菌及其对利福平耐药性的研究[J]. 中华实验和临床感染病杂志:电子版, 2015,9(4):524-527. |
| [36] | 罗兰波, 唐柳生. 痰液两种检验方法在肺结核诊断中的应用价值探讨[J]. 医药前沿, 2016,6(36):216-217. |
| [37] | 牛波, 刘建华, 曹丽洁, 等. Xpert MTB/RIF系统检测儿童肺结核灌洗液标本结果分析[J]. 皖南医学院学报, 2016,35(2):140-142. |
| [38] | 欧喜超, 池俊英, 赵雁林, 等. 半巢式全自动实时荧光定量核酸扩增检测在结核病诊断中的应用[C]// 科学研究与结核病防治高峰论坛论文汇编. 陕西延安:科学研究与结核病防治高峰论坛, 2014. |
| [39] | 司大. 抗酸染色法、固体培养法、Xpert MTB法对肺结核病人痰标本检测结果的比较分析[J]. 医药, 2016,1(1):213-215. |
| [40] |
田斌, 王孝君, 文岚, 等. Xpert MTB/RIF检测系统对肺结核临床诊断病例的应用价值[J]. 中国人兽共患病学报, 2016,32(9):798-801.
doi: 10.3969/j.issn.1002-2694.2016.09.007 |
| [41] | 韦宣彤. 对353例痰标本Xpert MTB/RIF检测和固体培养的结果分析[J]. 医药前沿, 2016,6(4):212-213. |
| [42] | 吴祥兵, 李娜, 蔡明明, 等. 应用Xpert MTB/RIF技术快速筛查耐多药肺结核[J]. 预防医学, 2017,29(7):754-756. |
| [43] | 杨丹丹. 利用GeneXpert检测结核分支杆菌及其利福平耐药性的研究[J]. 中国医药指南, 2017,15(12):175-176. |
| [44] | 杨慧娟, 马利, 陈连勇, 等. Xpert MTB/RIF技术对结核分枝杆菌检测和耐多药结核快速筛查的研究[J]. 中国卫生检验杂志, 2015,25(23):4056-4059. |
| [45] | 杨蕴华, 韩中波. Xpert MTB/RIF技术在结核病诊断及耐药检测中的应用[J]. 中国实用医药, 2015,10(7):195-196. |
| [46] | 叶鹏. 全自动实时荧光定量PCR系统在肺结核诊断和药敏试验检测中的应用价值[J]. 中国预防医学杂志, 2017,18(4):307-310. |
| [47] | 张灿强, 赖晓宇, 郭劼琳. Xpert MTB/RIF技术在肺结核诊断中的应用价值[J]. 现代医院, 2016,16(12):1783-1785. |
| [48] | 张国钦, 巨韩芳, 钟达, 等. Xpert MTB/RIF应用于可疑肺结核患者诊断效果分析[J]. 中国热带医学, 2017,17(3):266-269. |
| [49] |
张青, 薛冰茹, 李克诚. GeneXpert MTB/RIF系统对肺结核的诊断价值[J]. 检验医学, 2016,31(7):599-602.
doi: 10.3969/j.issn.1673-8640.2016.07.013 |
| [50] | 张燕, 李波. GeneXpert MTB/RIF快速检测法结核分支菌及其对利福平耐药性的临床价值评估[J]. 现代实用医学, 2016,28(10):1361-1362. |
| [51] | 张志学, 苍爱泽, 孙静. 应用GeneXpert MTB/RIF技术检测肺泡灌洗液对菌阴肺结核的临床诊断价值[J]. 结核病与肺部健康杂志, 2017,6(2):128-130. |
| [52] |
赵冰, 欧喜超, 夏辉, 等. Xpert MTB/RIF检测技术在结核病诊断中的应用评价[J]. 中国防痨杂志, 2014,36(6):462-466.
doi: 10.3969/j.issn.1000-6621.2014.06.011 |
| [53] | 钟丽云, 李静. Xpert MTB/RIF试验在县(区)基层结核病实验室诊断疑似肺结核中的应用价值[J]. 上海医药, 2017,38(5):41-43. |
| [54] | 周洪经, 郭明日, 冯爽, 等. Xpert MTB/RIF在快速诊断肺结核及利福平耐药中的临床应用[J]. 国际检验医学杂志, 2016,37(18):2568-2570. |
| [55] | 朱岩昆, 王宇, 靳晓伟, 等. 交叉引物核酸恒温扩增技术在基层实验室诊断肺结核的应用价值[J]. 中国防痨杂志, 2016,38(10):813-817. |
| [56] |
Steingart KR, Sohn H, Schiller I, et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults[J]. Cochrane Database Syst Rev, 2013(1): CD009593. doi: 10.1002/14651858.CD009593.
doi: 10.1002/14651858.CD004288.pub3 pmid: 26799160 |
| [1] | Qiu-yu LI,Ying LIANG,Ni-ni DAI,Yu-xiang WANG,Bo-tao ZHU,Rui WU,Hong ZHU,Yong-chang SUN. Hemophagocytic lymphohistiocytosis caused by hematogenous disseminated pulmonary tuberculosis: A case report [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1219-1223. |
| [2] | YUAN Chang-wei,WANG Ying-jin,ZHANG Shu-jie,SHEN Sheng-li,DUAN Hong-zhou. Clinical outcomes following microsurgery and endovascular embolization in the management of spinal dural arteriovenous fistula: A meta-analysis study [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 304-314. |
| [3] | Bao-qi ZENG,Shu-qing YU,Yao CHEN,Wei ZHAI,Bin LIU,Si-yan ZHAN,Feng SUN. Safety of biological valves for aortic valve replacement: A systematic review and meta-analysis [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 547-556. |
| [4] | Yi-ran ZHANG,Feng RAO,Wei PI,Pei-xun ZHANG,Bao-guo JIANG. Proximal femoral nails antirotation and dynamic hip screws for fixation of unstable intertrochanteric fractures of femur: A meta-analysis [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 493-500. |
| [5] | Shuang LIU,Yu-long GUO,Jing-yi YANG,Wei WANG,Jian XU. Efficacy of mesenchymal stem cells on systemic lupus erythematosus:a meta-analysis [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1014-1021. |
| [6] | SHI Hui-feng, ZHANG Jing-xu, ZHANG Rong, WANG Xiao-li. Prevalence of autism spectrum disorders in children aged 0-6 years in China: a meta-analysis [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 798-806. |
| [7] | MA Yan-yan, ZHANG Jing-jing, GAO Xue-mei. Treatment outcome evaluation of different mandibular advancements using oral appliance to treat obstructive sleep apnea and hyponea syndrome: a systematic review [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 691-699. |
| [8] | LI Zhi-xia, WU Shan-shan, YANG Zhi-rong, ZHAN Si-yan, SUN Feng. Impact of glucagon-like peptide-1 receptor agonists on nasopharyngitis and upper respiratory tract infection among patients with type 2 diabetes: a network meta-analysis [J]. Journal of Peking University(Health Sciences), 2016, 48(3): 454-459. |
| [9] | HUANG Yuan-Sheng, YANG Zhi-Rong, ZHAN Si-Yan. Comparison of simple pooling and bivariate model used in meta-analyses of diagnostic test accuracy published in Chinese journals [J]. Journal of Peking University(Health Sciences), 2015, 47(3): 483-488. |
| [10] | LIU Da-Jin, FENG Meng-Xian, LIU Min. Primary drug resistance of human immunodeficiency virus (HIV)among the treatment-naive individuals with HIV in China: a meta-analysis [J]. Journal of Peking University(Health Sciences), 2015, 47(3): 474-482. |
| [11] | WU Shan-Shan, ZHANG Yue-Lun, WANG Wei-Wei, CHEN Ru, SUN Feng, ZHAN Si-Yan- . Liver injury associated with treatment of multidrug-resistant tuberculosis: a syste-matic review and metaanalysis [J]. Journal of Peking University(Health Sciences), 2014, 46(3): 417-423. |
|
||