Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (6): 1122-1127. doi: 10.19723/j.issn.1671-167X.2021.06.019
Previous Articles Next Articles
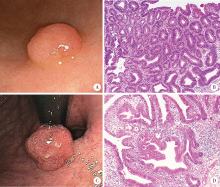
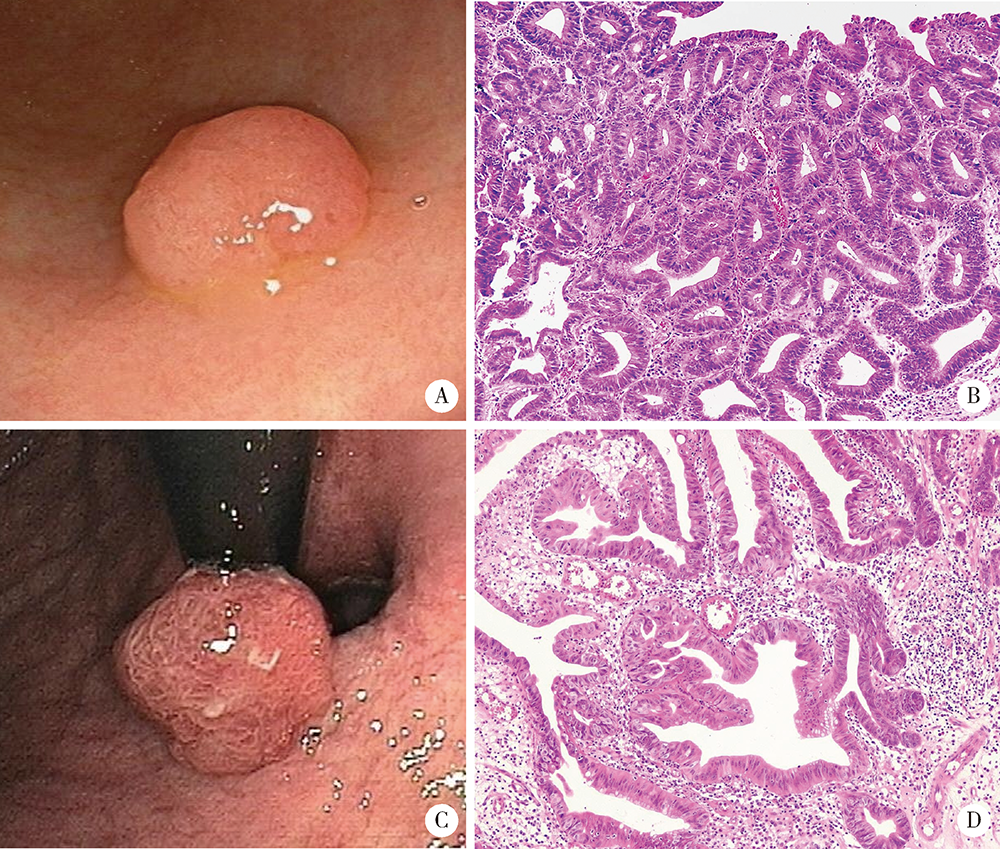
Analysis of endoscopic and pathological features of gastric adenomatous polyps and risk factors for canceration
NIU Zhan-yue,XUE Yan,ZHANG Jing,ZHANG He-jun,DING Shi-gang( )
)
- Department of Gastroenterology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R735.2
| [1] |
Carmack SW, Genta RM, Schuler CM, et al. The current spectrum of gastric polyps: A 1-year national study of over 120,000 patients[J]. Am J Gastroenterol, 2009, 104(6):1524-1532.
doi: 10.1038/ajg.2009.139 |
| [2] |
Corral JE, Keihanian T, Diaz LI, et al. Management patterns of gastric polyps in the United States[J]. Frontline Gastroenterol, 2019, 10(1):16-23.
doi: 10.1136/flgastro-2017-100941 |
| [3] |
Enestvedt BK, Chandrasekhara V, Ginsberg GG. Endoscopic ultrasonographic assessment of gastric polyps and endoscopic mucosal resection[J]. Curr Gastroenterol Rep, 2012, 14(6):497-503.
doi: 10.1007/s11894-012-0292-2 pmid: 23001857 |
| [4] |
Velázquez-Dohorn ME, López-Durand CF, Gamboa-Domínguez A. Changing trends in gastric polyps[J]. Rev Invest Clin, 2018, 70(1):40-45.
doi: 10.24875/RIC.17002430 pmid: 29513301 |
| [5] |
Castro R, Pimentel-Nunes P, Dinis-Ribeiro M. Evaluation and management of gastric epithelial polyps[J]. Best Pract Res Clin Gastroenterol, 2017, 31(4):381-387.
doi: S1521-6918(17)30060-4 pmid: 28842047 |
| [6] |
Chen WC, Wallace MB. Endoscopic management of mucosal lesions in the gastrointestinal tract[J]. Expert Rev Gastroenterol Hepatol, 2016, 10(4):481-495.
doi: 10.1586/17474124.2016.1122520 |
| [7] | Banks M, Graham D, Jansen M, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma[J]. Gut, 2019, 68(9):1545-1575. |
| [8] |
Laxén F, Sipponen P, Iham?ki T. Gastric polyps: their morphological and endoscopical characteristics and relation to gastric carcinoma[J]. Acta Pathol Microbiol Immunol Scand A, 1982, 90(3):221-228.
pmid: 7102316 |
| [9] |
Borch K, Skarsgard J, Franzen L, et al. Benign gastric polyps: Morphological and functional origin[J]. Dig Dis Sci, 2003, 48(7):1292-1297.
doi: 10.1023/A:1024150924457 |
| [10] |
Zhao G, Xue M, Hu Y, et al. How commonly is the diagnosis of gastric low grade dysplasia upgraded following endoscopic resection? A meta-analysis[J]. PLoS One, 2015, 10(7):e0132699.
doi: 10.1371/journal.pone.0132699 |
| [11] |
de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands[J]. Gastroenterology, 2008, 134(4):945-952.
doi: 10.1053/j.gastro.2008.01.071 |
| [12] |
Meining A, Riedl B, Stolte M. Features of gastritis predisposing to gastric adenoma and early gastric cancer[J]. J Clin Pathol, 2002, 55(10):770-773.
pmid: 12354805 |
| [13] |
Zhang H, Jin Z, Cui R, et al. Autoimmune metaplastic atrophic gastritis in chinese: A study of 320 patients at a large tertiary medical center[J]. Scand J Gastroenterol, 2017, 52(2):150-156.
doi: 10.1080/00365521.2016.1236397 |
| [14] |
Zhang H, Nie X, Song Z, et al. Hyperplastic polyps arising in autoimmune metaplastic atrophic gastritis patients: Is this a distinct clinicopathological entity[J]. Scand J Gastroenterol, 2018, 53(10/11):1186-1193.
doi: 10.1080/00365521.2018.1514420 |
| [15] |
Suzuki S, Gotoda T, Suzuki H, et al. Morphologic and histologic changes in gastric adenomas after Helicobacter pylori eradication: A long-term prospective analysis[J]. Helicobacter, 2015, 20(6):431-437.
doi: 10.1111/hel.12218 pmid: 25704290 |
| [16] |
Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection: the Maastricht V/Florence Consensus Report[J]. Gut, 2017, 66(1):6-30.
doi: 10.1136/gutjnl-2016-312288 pmid: 27707777 |
| [17] |
Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endosco-pic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline[J]. Endoscopy, 2015, 47(9):829-854.
doi: 10.1055/s-0034-1392882 pmid: 26317585 |
| [1] | Zhicun LI, Tianyu WU, Lei LIANG, Yu FAN, Yisen MENG, Qian ZHANG. Risk factors analysis and nomogram model construction of postoperative pathological upgrade of prostate cancer patients with single core positive biopsy [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 896-901. |
| [2] | Handong DING, Qin WANG, Guiyi LIAO, Zongyao HAO. Diagnosis and treatment of gastrointestinal bleeding after kidney transplantation [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 902-907. |
| [3] | Yuxuan TIAN,Mingjian RUAN,Yi LIU,Derun LI,Jingyun WU,Qi SHEN,Yu FAN,Jie JIN. Predictive effect of the dual-parametric MRI modified maximum diameter of the lesions with PI-RADS 4 and 5 on the clinically significant prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 567-574. |
| [4] | Kaifeng YAO,Mingjian RUAN,Derun LI,Yuxuan TIAN,Yuke CHEN,Yu FAN,Yi LIU. Diagnostic efficacy of targeted biopsy combined with regional systematic biopsy in prostate cancer in patients with PI-RADS 4-5 [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 575-581. |
| [5] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [6] | Ye YAN,Xiaolong LI,Haizhui XIA,Xuehua ZHU,Yuting ZHANG,Fan ZHANG,Ke LIU,Cheng LIU,Lulin MA. Analysis of risk factors for long-term overactive bladder after radical prostatectomy [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 589-593. |
| [7] | Yan CHEN,Kuangmeng LI,Kai HONG,Shudong ZHANG,Jianxing CHENG,Zhongjie ZHENG,Wenhao TANG,Lianming ZHAO,Haitao ZHANG,Hui JIANG,Haocheng LIN. Retrospective study on the impact of penile corpus cavernosum injection test on penile vascular function [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 680-686. |
| [8] | Bo PANG,Tongjun GUO,Xi CHEN,Huaqi GUO,Jiazhang SHI,Juan CHEN,Xinmei WANG,Yaoyan LI,Anqi SHAN,Hengyi YU,Jing HUANG,Naijun TANG,Yan WANG,Xinbiao GUO,Guoxing LI,Shaowei WU. Personal nitrogen oxides exposure levels and related influencing factors in adults over 35 years old in Tianjin and Shanghai [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 700-707. |
| [9] | Jing HE,Zhongze FANG,Ying YANG,Jing LIU,Wenyao MA,Yong HUO,Wei GAO,Yangfeng WU,Gaoqiang XIE. Relationship between lipid metabolism molecules in plasma and carotid atheroscle-rotic plaques, traditional cardiovascular risk factors, and dietary factors [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 722-728. |
| [10] | Shan CAI,Yihang ZHANG,Ziyue CHEN,Yunfe LIU,Jiajia DANG,Di SHI,Jiaxin LI,Tianyu HUANG,Jun MA,Yi SONG. Status and pathways of factors influencing physical activity time among elementary and junior high school students in Beijing [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 403-410. |
| [11] | Zuhong ZHANG,Tianjiao CHEN,Jun MA. Associations between puberty timing and cardiovascular metabolic risk factors among primary and secondary students [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 418-423. |
| [12] | Yuting LIN,Huali WANG,Yu TIAN,Litong GONG,Chun CHANG. Factors influencing cognitive function among the older adults in Beijing [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 456-461. |
| [13] | Jinrong ZHU,Yana ZHAO,Wei HUANG,Weiwei ZHAO,Yue WANG,Song WANG,Chunyan SU. Clinical characteristics of COVID-19 infection in patients undergoing hemodialysis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 267-272. |
| [14] | Zhanhong LAI,Jiachen LI,Zelin YUN,Yonggang ZHANG,Hao ZHANG,Xiaoyan XING,Miao SHAO,Yuebo JIN,Naidi WANG,Yimin LI,Yuhui LI,Zhanguo LI. A unicenter real-world study of the correlation factors for complete clinical response in idiopathic inflammatory myopathies [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 284-292. |
| [15] | Xiaoqian SI,Xiujuan ZHAO,Fengxue ZHU,Tianbing WANG. Risk factors for acute respiratory distress syndrome in patients with traumatic hemorrhagic shock [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 307-312. |
|
||