Journal of Peking University (Health Sciences) ›› 2026, Vol. 58 ›› Issue (1): 10-21. doi: 10.19723/j.issn.1671-167X.2026.01.002
Previous Articles Next Articles
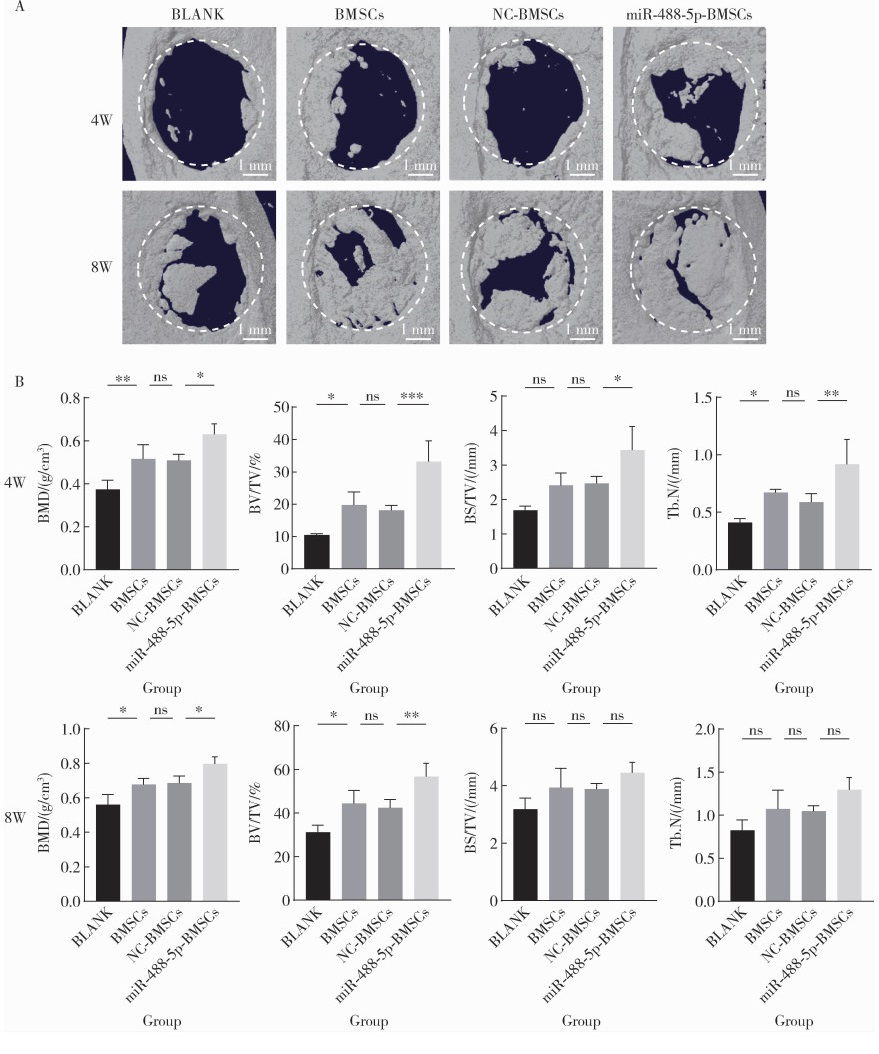
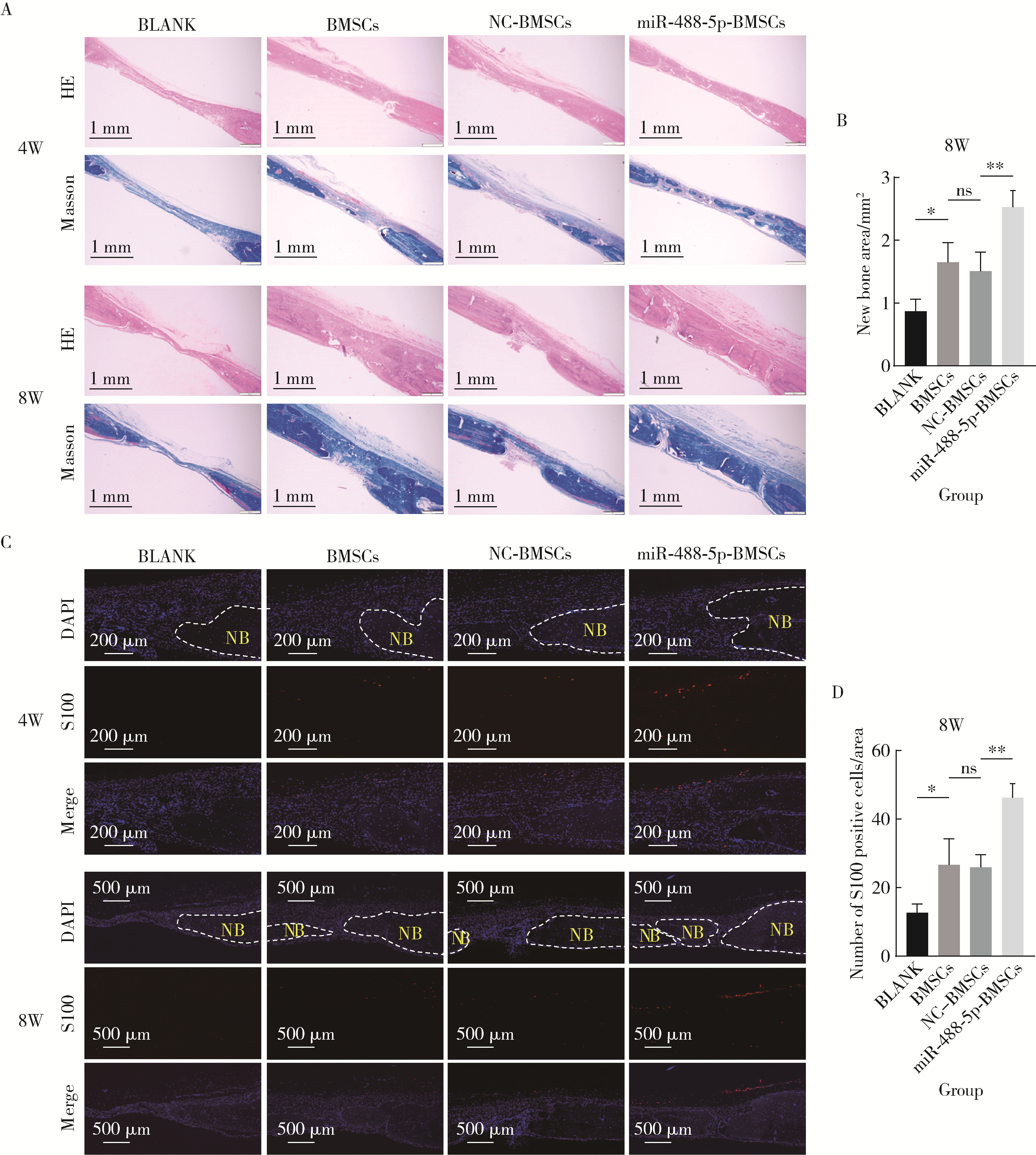
miR-488-5p promotes osteogenic and neurogenic differentiation of rat bone marrow mesenchymal stem cells and enhances neuralized bone regeneration
Liting ZENG1, Kaiyuan CHENG1,2, Zhongning LIU1, Jian LI1, Jingwen YANG1,*( ), Ting JIANG1,*(
), Ting JIANG1,*( )
)
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices, Beijing 100081, China
2. Department Ⅰ of Prosthodontics, Tianjin Stomatological Hospital, School of Medicine, Nankai University, Tianjin Key Laboratory of Oral and Maxillofacial Function Reconstruction, Tianjin 300041, China
CLC Number:
- R68
| 1 |
doi: 10.1038/s41413-023-00302-8 |
| 2 |
|
| 3 |
doi: 10.1021/acsami.4c16786 |
| 4 |
|
| 5 |
doi: 10.1186/s13287-021-02433-3 |
| 6 |
doi: 10.1016/j.biomaterials.2021.120995 |
| 7 |
doi: 10.1002/advs.202206155 |
| 8 |
|
| 9 |
doi: 10.1016/j.mtbio.2024.100985 |
| 10 |
doi: 10.1111/nyas.13206 |
| 11 |
doi: 10.3390/ijms16048227 |
| 12 |
doi: 10.1002/jbm4.10213 |
| 13 |
doi: 10.1016/j.addr.2015.05.007 |
| 14 |
doi: 10.1002/jnr.25181 |
| 15 |
doi: 10.3390/ijms21041252 |
| 16 |
doi: 10.1111/nyas.14120 |
| 17 |
程凯远, 张美子, 刘中宁, 等. 下牙槽神经离断对下颌骨骨质和拔牙创愈合的影响研究[J]. 口腔颌面修复学杂志, 2025, 26(2): 105- 115.
|
| 18 |
doi: 10.1002/advs.202003390 |
| 19 |
doi: 10.1371/journal.pone.0044768 |
| 20 |
doi: 10.1039/C9TB00025A |
| 21 |
|
| 22 |
|
| 23 |
doi: 10.1007/s11033-024-09577-4 |
| 24 |
doi: 10.1159/000095985 |
| 25 |
doi: 10.1016/j.biomaterials.2021.121216 |
| 26 |
doi: 10.3390/polym16213039 |
| 27 |
doi: 10.1186/s40824-023-00422-6 |
| 28 |
doi: 10.1021/acs.biomac.3c00279 |
| 29 |
丁梦, 李强, 李筱叶, 等. 光固化水凝胶负载骨髓间充质干细胞修复大鼠颅骨缺损[J]. 口腔疾病防治, 2024, 32(5): 330- 340.
|
| 30 |
doi: 10.1016/j.mtbio.2023.100882 |
| 31 |
|
| [1] | Chunhui SHENG, Xiao ZHANG, Longwei LV, Yongsheng ZHOU. Exosome derived from human adipose-derived mesenchymal stem cells prevented bone loss induced by estrogen deficiency [J]. Journal of Peking University (Health Sciences), 2025, 57(2): 217-226. |
| [2] | Yibo HU, Weijia LYU, Wei XIA, Yihong LIU. Hydrodynamic finite element analysis of biological scaffolds with different pore sizes for cell growth and osteogenic differentiation [J]. Journal of Peking University (Health Sciences), 2025, 57(1): 97-105. |
| [3] | Ting SHUAI, Yanyan GUO, Chunping LIN, Xiaomei HOU, Chanyuan JIN. Knockdown of NPTX1 promotes osteogenic differentiation of human bone marrow mesenchymal stem cells [J]. Journal of Peking University (Health Sciences), 2025, 57(1): 7-12. |
| [4] | SHUAI Ting,LIU Juan,GUO Yan-yan,JIN Chan-yuan. Knockdown of long non-coding RNA MIR4697 host gene inhibits adipogenic differentiation in bone marrow mesenchymal stem cells [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 320-326. |
| [5] | Jing XIE,Yu-ming ZHAO,Nan-quan RAO,Xiao-tong WANG,Teng-jiao-zi FANG,Xiao-xia LI,Yue ZHAI,Jing-zhi LI,Li-hong GE,Yuan-yuan WANG. Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 900-906. |
| [6] | Xia LIU,Ying ni LI,Xiao li SUN,Qing lin PENG,Xin LU,Guo chun WANG. Effects of integrin metalloproteinases on osteogenic differentiation [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 962-967. |
|
||