Journal of Peking University (Health Sciences) ›› 2026, Vol. 58 ›› Issue (1): 43-49. doi: 10.19723/j.issn.1671-167X.2026.01.006
Previous Articles Next Articles
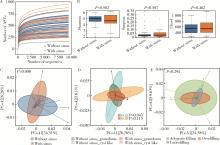
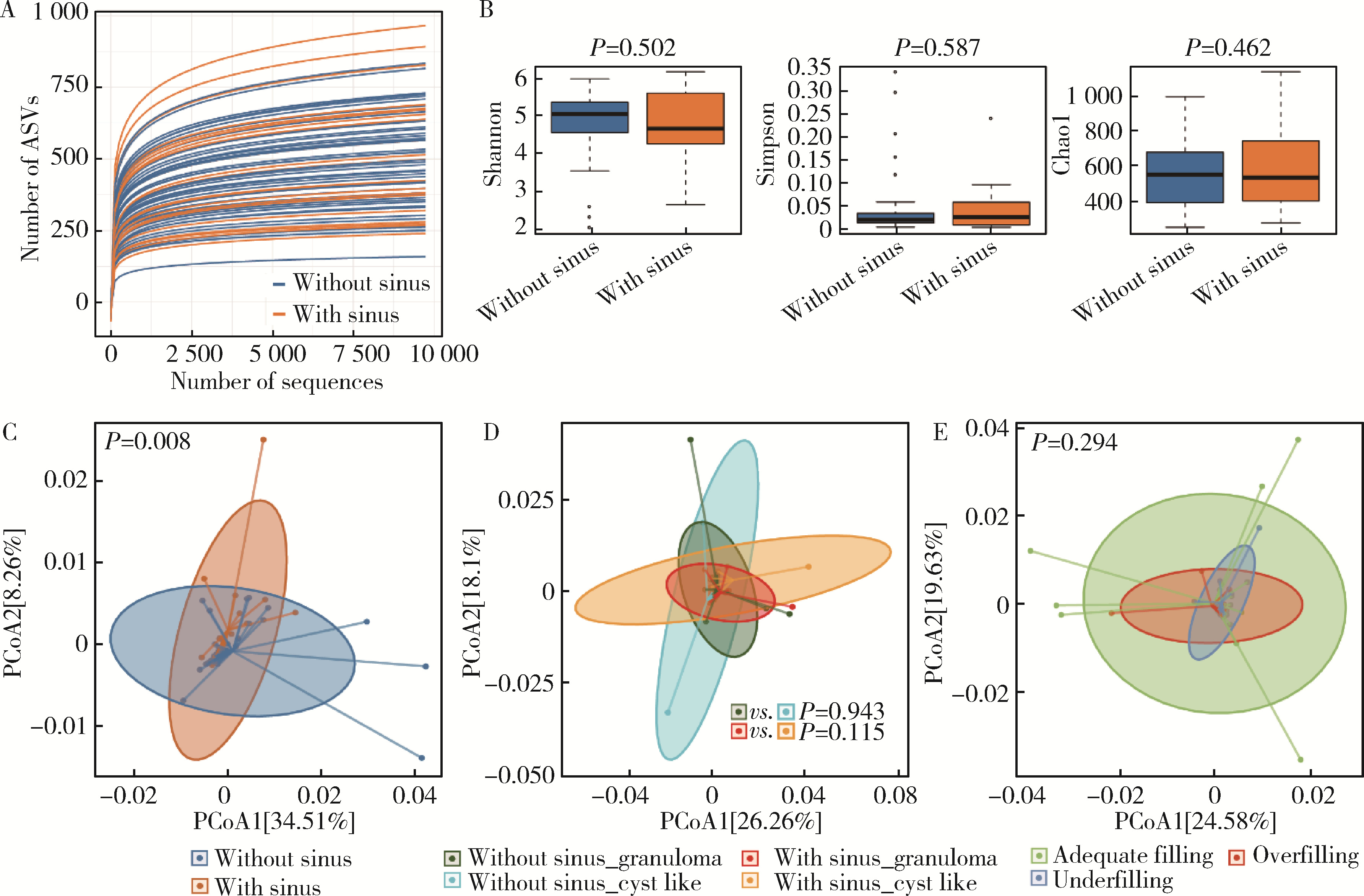
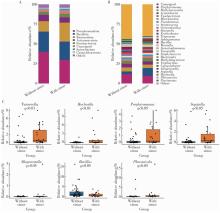
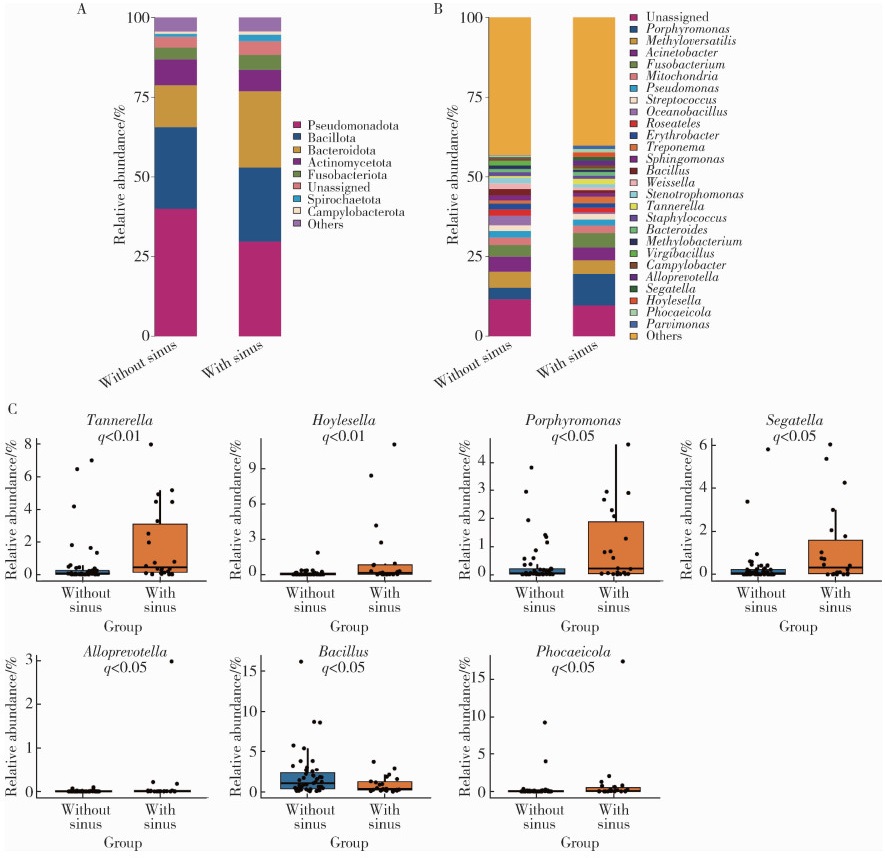
Microbial communities in extraradicular infections of post-treatment apical periodontitis without or with sinus tracts
Rentao TANG, Liuchang YANG, Jie NIE*( ), Xiaoyan WANG
), Xiaoyan WANG
- Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices, Beijing 100081, China
CLC Number:
- R781.3
| 1 |
doi: 10.1016/j.tripleo.2004.10.005 |
| 2 |
doi: 10.1111/j.1365-2591.2006.01099.x |
| 3 |
doi: 10.1111/iej.13457 |
| 4 |
doi: 10.3389/fcimb.2021.798367 |
| 5 |
doi: 10.3389/fcimb.2022.980157 |
| 6 |
doi: 10.1097/00004770-200312000-00003 |
| 7 |
doi: 10.1111/j.1365-2591.2011.01872.x |
| 8 |
doi: 10.1016/j.jdent.2024.105496 |
| 9 |
漆正楠, 尹君, 唐子圣, 等. 有窦型与无窦型根尖周炎微生物群落比较[J]. 牙体牙髓牙周病学杂志, 2016, 26(1): 1- 6.
|
| 10 |
doi: 10.1016/S0030-4220(80)80015-6 |
| 11 |
doi: 10.1111/j.1365-2591.2008.01397.x |
| 12 |
doi: 10.1111/iej.12428 |
| 13 |
doi: 10.1111/iej.13512 |
| 14 |
doi: 10.1038/s41587-019-0209-9 |
| 15 |
doi: 10.1016/j.joen.2024.07.016 |
| 16 |
doi: 10.1016/j.joen.2022.11.015 |
| 17 |
李昱志, 苏旭, 陈晓涛, 等. 基于16S rDNA测序的慢性牙髓炎及根尖周炎感染根管内菌群多样性研究[J]. 安徽医科大学学报, 2024, 59(9): 1669- 1674.
|
| 18 |
doi: 10.1111/iej.70011 |
| 19 |
doi: 10.3390/biom12121737 |
| 20 |
doi: 10.1007/s00784-020-03510-2 |
| 21 |
doi: 10.1111/iej.13911 |
| 22 |
doi: 10.1038/nrmicro2337 |
| 23 |
doi: 10.1111/iej.14218 |
| 24 |
doi: 10.1111/jre.13363 |
| 25 |
doi: 10.1007/s10096-009-0790-9 |
| 26 |
doi: 10.1007/s00784-025-06592-y |
| [1] | Jing-cheng XIE,Xiao-dong CHEN,Jun YANG. Diagnosis and surgical treatment of tethered cord syndrome accompanied by congenital dermal sinus tract in adults [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1163-1166. |
| [2] | Jia-he ZHANG, Jia-qi SHI, Zhang-jian CHEN, Guang JIA. Effects of nano titanium dioxide on gut microbiota based on human digestive tract microecology simulation system in vitro [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 468-476. |
| [3] | ZHU Shi-wei, LIU Zuo-jing, LI Mo, ZHU Huai-qiu, DUAN Li-ping. Comparison of gut microbiotal compositional analysis of patients with irritable bowel syndrome through different bioinformatics pipelines [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 231-238. |
|
||