Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (3): 402-408. doi: 10.19723/j.issn.1671-167X.2019.03.005
Previous Articles Next Articles
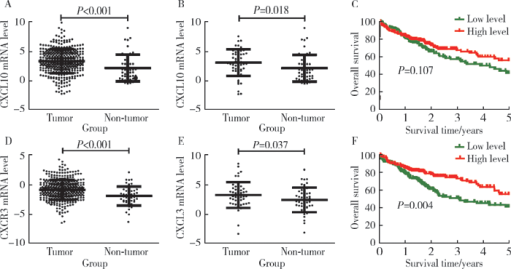
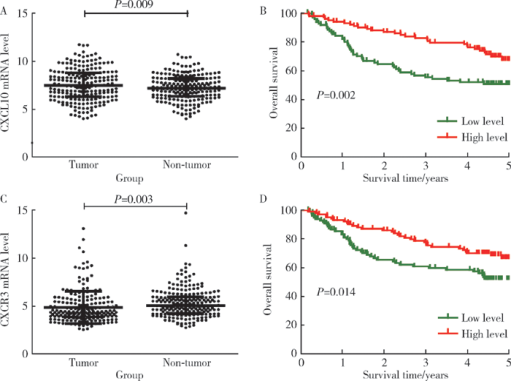
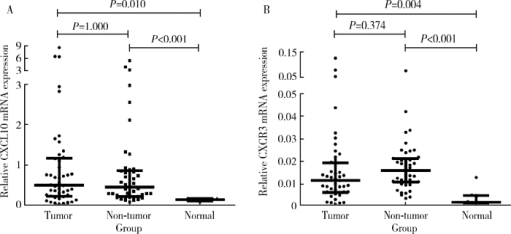
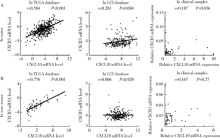
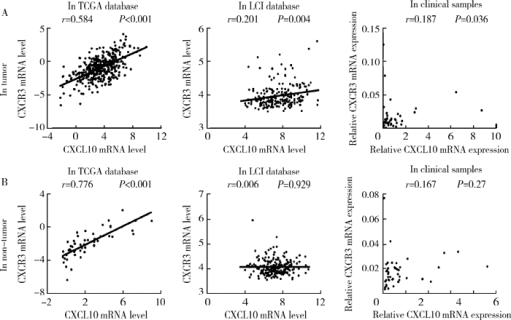
Expression and clinical significance of chemokine CXCL10 and its receptor CXCR3 in hepatocellular carcinoma
Jing ZHANG,Jie CHEN,Gui-wen GUAN,Ting ZHANG,Feng-min LU( ),Xiang-mei CHEN△(
),Xiang-mei CHEN△( )
)
- Department of Microbiology & Infectious Disease Center, Peking University School of Basic Medical Sciences, Beijing 100191, China
CLC Number:
- R393
| [1] |
Joliat GR, Allemann P, Labgaa I , et al. Treatment and outcomes of recurrent hepatocellular carcinomas[J]. Langenbecks Arch Surg, 2017,402(5):737-744.
doi: 10.1007/s00423-017-1582-9 |
| [2] |
El-Serag HB, Rudolph KL . Hepatocellular carcinoma: epidemio-logy and molecular carcinogenesis[J]. Gastroenterology, 2007,132(7):2557-2576.
doi: 10.1053/j.gastro.2007.04.061 |
| [3] |
Hong TP, Gow P, Fink M , et al. Novel population-based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed[J]. Hepatology, 2016,63(4):1205-1212.
doi: 10.1002/hep.28267 |
| [4] | Elia G, Fallahi P . Hepatocellular carcinoma and CXCR3 chemokines: a narrative review[J]. Clin Ter, 2017,168(1):e37-e41. |
| [5] | 中华人民共和国卫生部. 原发性肝癌诊疗规范(2011年版)[J]. 临床肝胆病杂志, 2011,27(11):1141-1159. |
| [6] |
Kamil F, Rowe JH . How does the tumor microenvironment play a role in hepatobiliary tumors?[J]. J Gastrointest Oncol, 2018,9(1):180-195.
doi: 10.21037/jgo |
| [7] |
Tokunaga R, Zhang W, Naseem M , et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation:a target for novel cancer therapy[J]. Cancer Treat Rev, 2018,63:40-47.
doi: 10.1016/j.ctrv.2017.11.007 |
| [8] | Ren T, Zhu L, Cheng M . CXCL10 accelerates EMT and metastasis by MMP-2 in hepatocellular carcinoma[J]. Am J Transl Res, 2017,9(6):2824-2837. |
| [9] | Hirano S, Iwashita Y, Sasaki A , et al. Increased mRNA expression of chemokines in hepatocellular carcinoma with tumor-infiltrating lymphocytes[J]. J Gastroenterol Hepatol, 2007,22(5):690-696. |
| [10] |
Chew V, Chen J, Lee D , et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma[J]. Gut, 2012,61(3):427-438.
doi: 10.1136/gutjnl-2011-300509 |
| [11] | Ding Q, Xia Y, Ding S , et al. An alternatively spliced variant of CXCR3 mediates the metastasis of CD133 + liver cancer cells induced by CXCL9 [J]. Oncotarget, 2016,7(12):14405-14414. |
| [12] | 任颖, 阚云珍, 孔令非 . 靶向沉默CXCR3对肝癌细胞恶性增殖的作用研究[J]. 中华肝脏病杂志, 2018,26(7):508-512. |
| [13] | Billottet C, Quemener C, Bikfalvi A . CXCR3, a double-edged sword in tumor progression and angiogenesis[J]. Biochim Biophys Acta, 2013,1836(2):287-295. |
| [1] | Xiaodong CHAI,Ziwen SUN,Haishuang LI,Liangyi ZHU,Xiaodan LIU,Yantao LIU,Fei PEI,Qing CHANG. Clinicopathological characteristics of the CD8+ T lymphocytes infiltration and its mechanism in distinct molecular subtype of medulloblastoma [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 512-518. |
| [2] | Fang CHENG,Shao-ying YANG,Xing-xing FANG,Xuan WANG,Fu-tao ZHAO. Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1074-1078. |
| [3] | ZHONG Hua,XU Li-ling,BAI Ming-xin,SU Yin. Effect of chemokines CXCL9 and CXCL10 on bone erosion in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1026-1031. |
| [4] | PANG Yong,ZHANG Sha,YANG Hua,ZHOU Rou-li. Serum LAPTM4B-35 protein as a novel diagnostic marker for hepatocellular carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 710-715. |
| [5] | Bo MA,Zhi-hua TIAN,Li QU,Yue-xiang LIU,Hong ZHANG,Hui-rong DING. Establishment and gene expression analysis of drug-resistant cell lines in hepatocellular carcinoma induced by sorafenib [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 207-213. |
| [6] | Xin-yun YAO,Xiao-min GAO,Xiao-ying ZOU,Lin YUE. Role of endocytosis in cell surface CXC chemokine receptor 4 expression of stem cells from apical papilla [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 893-899. |
| [7] | Yun-bo XIE,Ji-yuan ZHANG,Mei-ling DU,Fan-ping MENG,Jun-liang FU,Li-min LIU,Song-shan WANG,Rui QU,Fang LIAN,Fei QIAO,Yang-liu CHEN,Ying-ying GAO,Ruo-nan XU,Ming SHI,Fu-sheng WANG. Efficacy and peripheral immunity analysis of allogeneic natural killer cells therapy in patients with hepatocellular carcinoma [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 591-595. |
| [8] | LIU Hong-jiang, SHI Lian-jie, HU Fan-lei, YAO Hai-hong, LI Zhan-guo, JIAYuan. Increased serum C-C chemokine ligand 19 levels correlated with B cell abnormalities in systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 829-834. |
| [9] | SHI Lian-jie, LI Jian-hong, HU Fan-lei, LI Min, ZHANG Jie, LI Jiang-tao, LI Zhan-guo. Clinical significance of serum C-C chemokine ligand 19 levels in patients with rheumatoid arthritis [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 667-671. |
| [10] | ZHANG Xiao-wei, DUN Yao-jun, TANG Xu, YIN Hua-qi, HU Zhi-ping, ZHAO Yong-ping, XU Tao, LI Qing. Expression of chemokine like factor-like myelin and lymphocyte and related proteins for vesicle trafficking and membrane link transmembrane domaincontaining protein 2 in rats with varicocele [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 579-583. |
| [11] | WEN Quan, ZHAO Yu-ming, WANG Yuan-yuan, WANG Xu, LING Long, GE Li-hong. Effects of stromal cell-derived factor-1 on proliferation, migration, and odontoblastic differentiation of human dental pulp stem cells [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 23-29. |
| [12] | 欧Meng-恩 , ZHANG Xiao, LIU Yun-Song, GE Yan-Jun, ZHOU Yong-Sheng. Ectopic osteogenesis of stromal cell-derived factor 1 combined with simvastatin loaded collagen scaffold in vivo [J]. Journal of Peking University(Health Sciences), 2015, 47(1): 47-51. |
|
||