Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (6): 1026-1031. doi: 10.19723/j.issn.1671-167X.2021.06.003
Previous Articles Next Articles
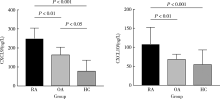
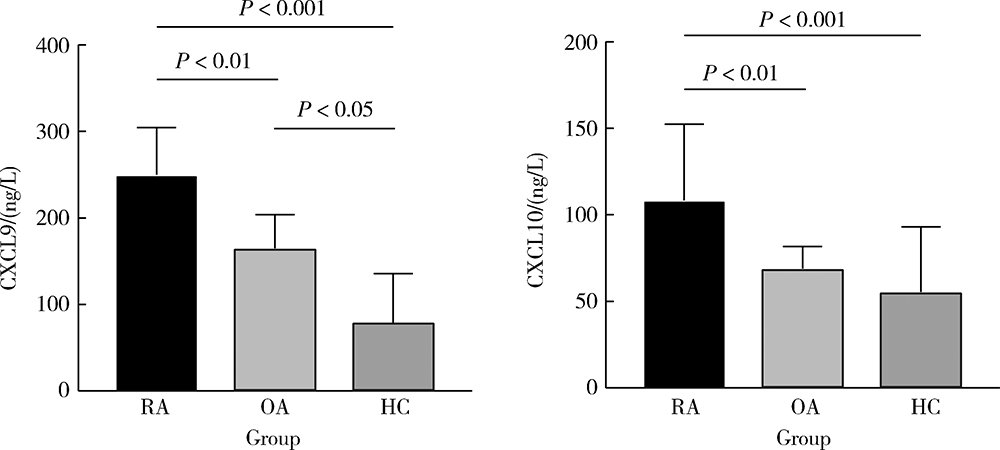
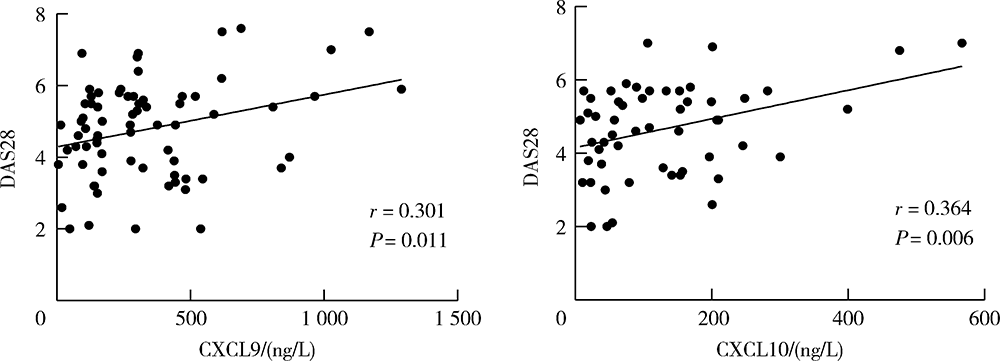
Effect of chemokines CXCL9 and CXCL10 on bone erosion in patients with rheumatoid arthritis
ZHONG Hua,XU Li-ling,BAI Ming-xin,SU Yin( )
)
- Beijing Key Laboratory for Rheumatism Mechanism and Immune Diagnosis, Beijing 100044, China
CLC Number:
- R593.22
| [1] |
Sparks JA. Rheumatoid arthritis [J]. Ann Intern Med, 2019, 170(1): ITC1-ITC16.
doi: 10.7326/AITC201901010 |
| [2] |
Zhu H, Li R, Da Z, et al. Remission assessment of rheumatoid arthritis in daily practice in China: A cross-sectional observational study[J]. Clin Rheumatol, 2018, 37(3):597-605.
doi: 10.1007/s10067-017-3850-z |
| [3] |
Zhou Y, Wang X, An Y, et al. Disability and health-related quality of life in Chinese patients with rheumatoid arthritis: A cross-sectional study[J]. Int J Rheum Dis, 2018, 21(9):1709-1715.
doi: 10.1111/apl.2018.21.issue-9 |
| [4] |
Poeta VM, Massara M, Capucetti A, et al. Chemokines and chemokine receptors: new targets for cancer immunotherapy[J]. Front Immunol, 2019, 10:379.
doi: 10.3389/fimmu.2019.00379 |
| [5] |
Susek KH, Karvouni M, Alici E, et al. The role of CXC chemokine receptors 1-4 on immune cells in the tumor microenvironment[J]. Front Immunol, 2018, 9:2159.
doi: 10.3389/fimmu.2018.02159 |
| [6] |
Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation: A target for novel cancer therapy[J]. Cancer Treat Rev, 2018, 63:40-47.
doi: S0305-7372(17)30199-8 pmid: 29207310 |
| [7] |
McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis[J]. N Engl J Med, 2011, 365(23):2205-2219.
doi: 10.1056/NEJMra1004965 |
| [8] |
Muntyanu A, Abji F, Liang K, et al. Differential gene and protein expression of chemokines and cytokines in synovial fluid of patients with arthritis[J]. Arthritis Res Ther, 2016, 18(1):296.
doi: 10.1186/s13075-016-1196-6 |
| [9] |
Antonelli A, Ferrari SM, Giuggioli D, et al. Chemokine (C-X-C motif) ligand CXCL10 in autoimmune diseases[J]. Autoimmun Rev, 2014, 13(3):272-280.
doi: 10.1016/j.autrev.2013.10.010 |
| [10] |
Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative[J]. Arthritis Rheum, 2010, 62(9):2569-2581.
doi: 10.1002/art.27584 |
| [11] |
Zhang W, Doherty M, Peat G, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis[J]. Ann Rheum Dis, 2010, 69(3):483-489.
doi: 10.1136/ard.2009.113100 pmid: 19762361 |
| [12] |
Prevoo ML, van’t Hof MA, Kuper HH, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis[J]. Arthritis Rheum, 1995, 38(1):44-48.
doi: 10.1002/art.v38:1 |
| [13] |
Fransen J, van Riel PL. The disease activity score and the EULAR response criteria [J]. Rheum Dis Clin North Am, 2009, 35(4): 745-757, vii-viii.
doi: 10.1016/j.rdc.2009.10.001 |
| [14] |
Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis[J]. Arthritis Rheum, 1988, 31(3):315-324.
doi: 10.1002/(ISSN)1529-0131 |
| [15] | Ostergaard M, Peterfy C, Conaghan P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system[J]. J Rheumatol, 2003, 30(6):1385-1386. |
| [16] |
Bruyn GA, Hanova P, Iagnocco A, et al. Ultrasound definition of tendon damage in patients with rheumatoid arthritis. Results of a OMERACT consensus-based ultrasound score focusing on the diagnostic reliability[J]. Ann Rheum Dis, 2014, 73(11):1929-1934.
doi: 10.1136/annrheumdis-2013-203596 |
| [17] |
Zeidler H. The need to better classify and diagnose early and very early rheumatoid arthritis[J]. J Rheumatol, 2012, 39(2):212-217.
doi: 10.3899/jrheum.110967 |
| [18] |
Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: Positioning cells for host defense and immunity[J]. Annu Rev Immunol, 2014, 32:659-702.
doi: 10.1146/annurev-immunol-032713-120145 pmid: 24655300 |
| [19] |
Korniejewska A, McKnight AJ, Johnson Z, et al. Expression and agonist responsiveness of CXCR3 variants in human T lymphocytes[J]. Immunology, 2011, 132(4):503-515.
doi: 10.1111/j.1365-2567.2010.03384.x pmid: 21255008 |
| [20] |
Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses[J]. Adv Immunol, 2007, 96:41-101.
pmid: 17981204 |
| [21] |
Farber JM. Mig and IP-10: CXC chemokines that target lymphocytes[J]. J Leukoc Biol, 1997, 61(3):246-257.
doi: 10.1002/jlb.1997.61.issue-3 |
| [22] |
Kwak HB, Ha H, Kim HN, et al. Reciprocal cross-talk between RANKL and interferon-gamma-inducible protein 10 is responsible for bone-erosive experimental arthritis[J]. Arthritis Rheum, 2008, 58(5):1332-1342.
doi: 10.1002/(ISSN)1529-0131 |
| [23] |
Kraan MC, Patel DD, Haringman JJ, et al. The development of clinical signs of rheumatoid synovial inflammation is associated with increased synjournal of the chemokine CXCL8 (interleukin-8)[J]. Arthritis Res, 2001, 3(1):65-71.
pmid: 11178128 |
| [1] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [2] | Huina HUANG,Jing ZHAO,Xiangge ZHAO,Ziran BAI,Xia LI,Guan WANG. Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 519-525. |
| [3] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [4] | Xue ZOU,Xiao-juan BAI,Li-qing ZHANG. Effectiveness of tofacitinib combined with iguratimod in the treatment of difficult-to-treat moderate-to-severe rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1013-1021. |
| [5] | Qi WU,Yue-ming CAI,Juan HE,Wen-di HUANG,Qing-wen WANG. Correlation between dyslipidemia and rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 982-992. |
| [6] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Correlation analysis between body mass index and clinical characteristics of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 993-999. |
| [7] | Yin-ji JIN,Lin SUN,Jin-xia ZHAO,Xiang-yuan LIU. Significance of IgA isotype of anti-v-raf murine sarcoma viral oncogene homologue B1 antibody in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 631-635. |
| [8] | Wen-xin CAI,Shi-cheng LI,Yi-ming LIU,Ru-yu LIANG,Jing LI,Jian-ping GUO,Fan-lei HU,Xiao-lin SUN,Chun LI,Xu LIU,Hua YE,Li-zong DENG,Ru LI,Zhan-guo LI. A cross-sectional study on the clinical phenotypes of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1068-1073. |
| [9] | Fang CHENG,Shao-ying YANG,Xing-xing FANG,Xuan WANG,Fu-tao ZHAO. Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1074-1078. |
| [10] | Rui LIU,Jin-xia ZHAO,Liang YAN. Clinical characteristics of patients with rheumatoid arthritis complicated with venous thrombosis of lower extremities [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1079-1085. |
| [11] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Cross-sectional study on quality of life and disease activity of rheumatoid arthritis patients [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1086-1093. |
| [12] | GAO Chao,CHEN Li-hong,WANG Li,YAO Hong,HUANG Xiao-wei,JIA Yu-bo,LIU Tian. Validation of the Pollard’s classification criteria (2010) for rheumatoid arthritis patients with fibromyalgia [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 278-282. |
| [13] | LUO Liang,HUO Wen-gang,ZHANG Qin,LI Chun. Clinical characteristics and risk factors of rheumatoid arthritis with ulcerative keratitis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1032-1036. |
| [14] | LOU Xue,LIAO Li,LI Xing-jun,WANG Nan,LIU Shuang,CUI Ruo-mei,XU Jian. Methylation status and expression of TWEAK gene promoter region in peripheral blood of patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1020-1025. |
| [15] | ZHANG Lu,HU Xiao-hong,CHEN Cheng,CAI Yue-ming,WANG Qing-wen,ZHAO Jin-xia. Analysis of cervical instability and clinical characteristics in treatment-naive rheumatoid arthritis patients [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1049-1054. |
|
||