Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (3): 519-525. doi: 10.19723/j.issn.1671-167X.2024.03.020
Previous Articles Next Articles
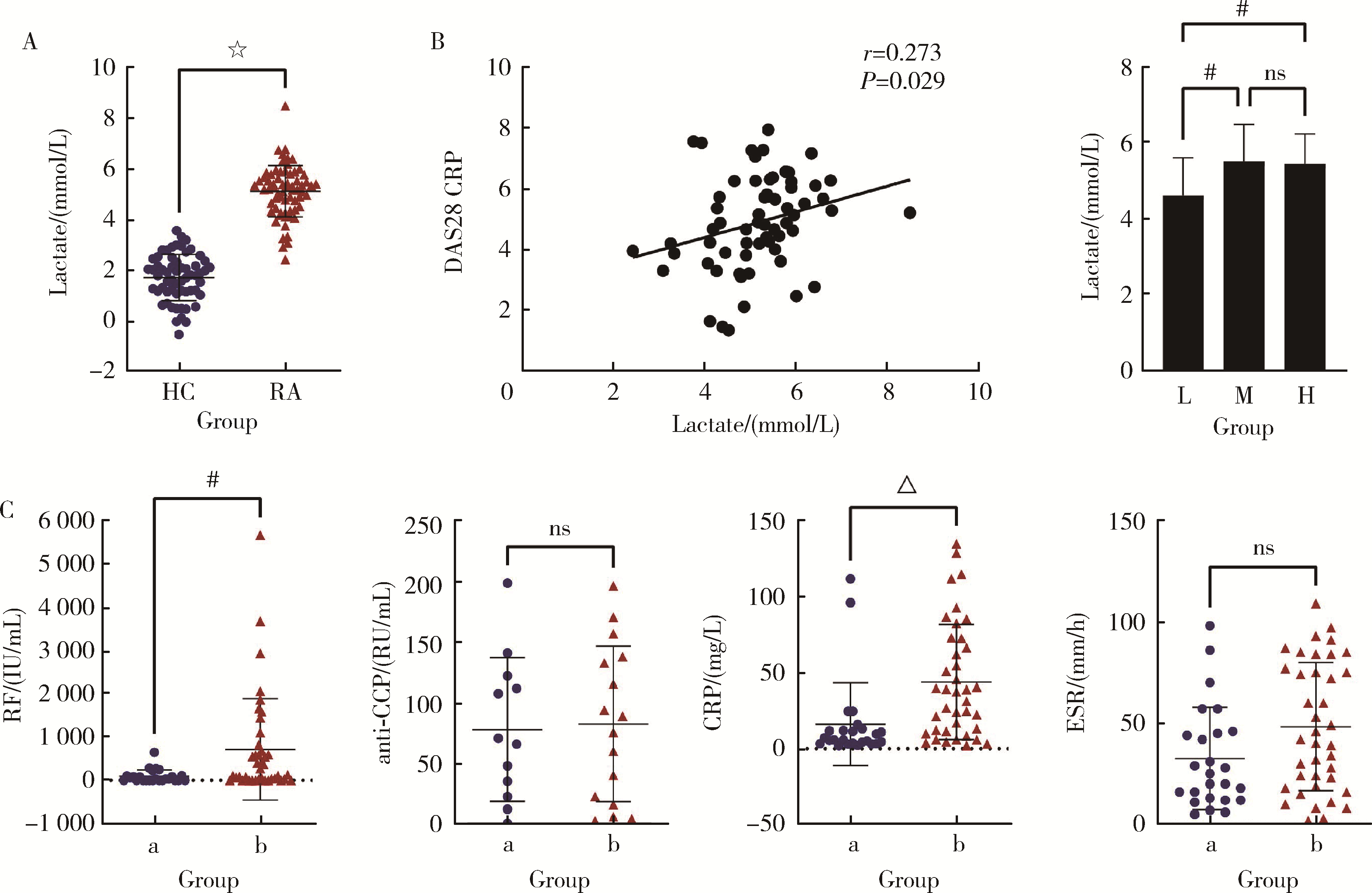
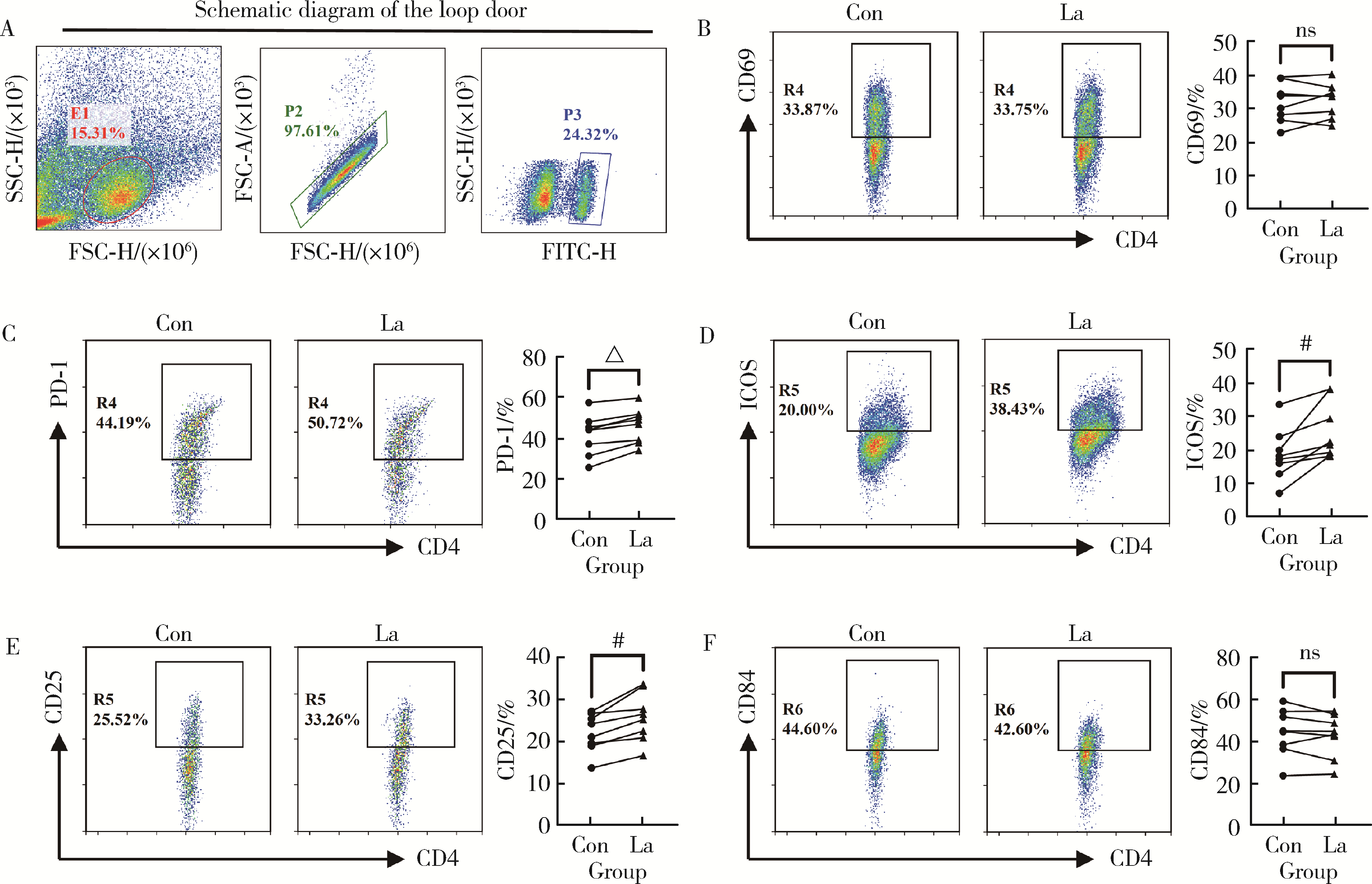
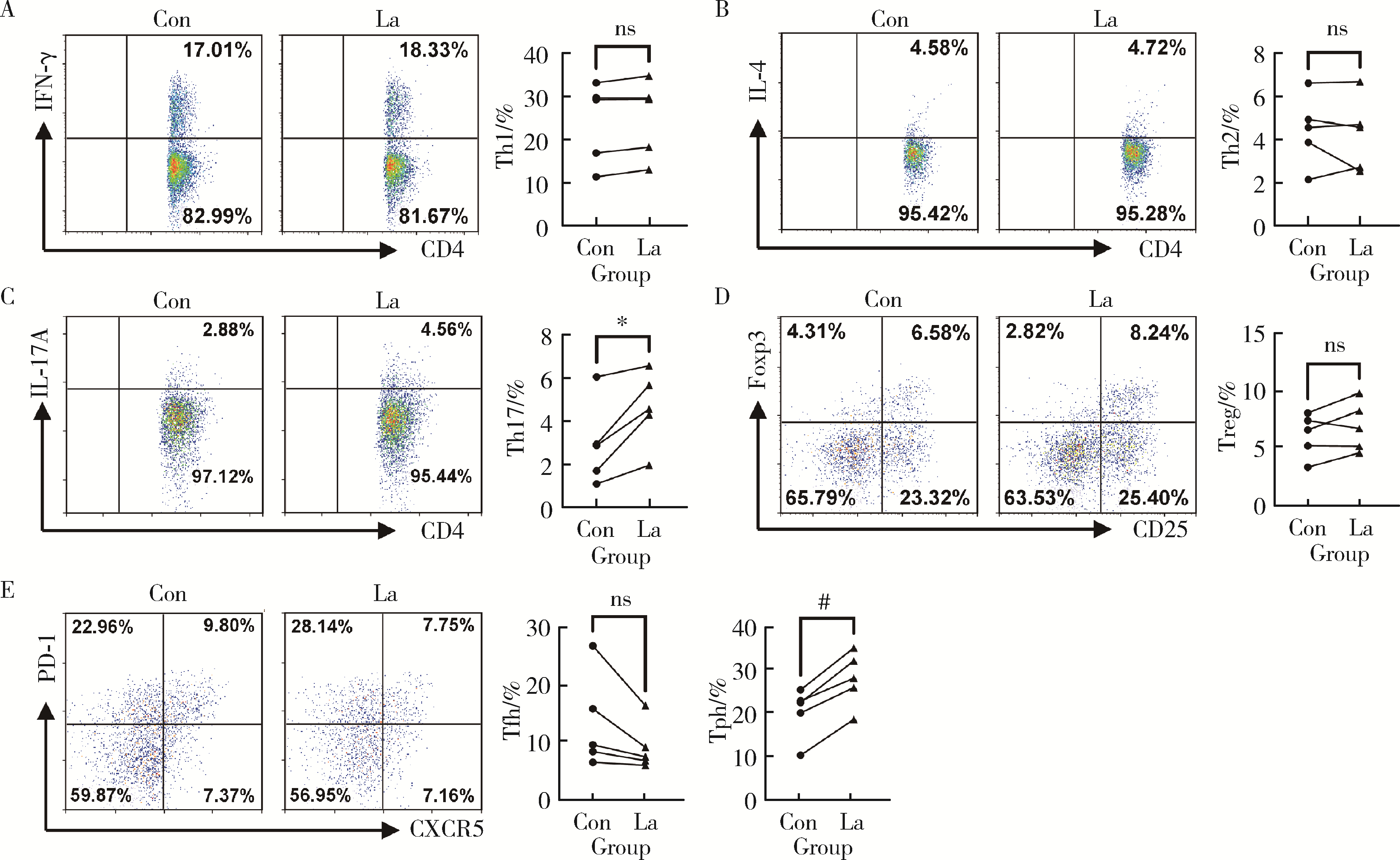
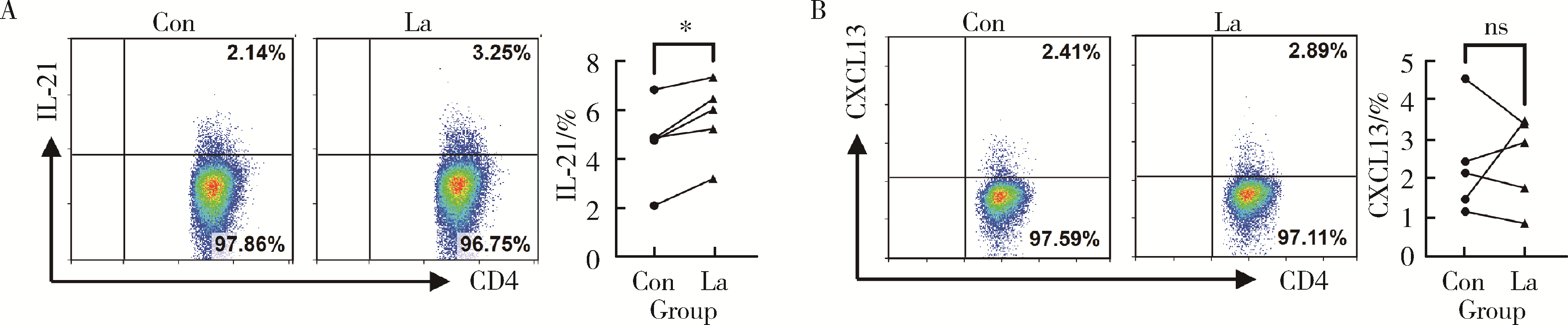
Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis
Huina HUANG1,Jing ZHAO2,Xiangge ZHAO1,Ziran BAI1,Xia LI1,Guan WANG1,*( )
)
- 1. Department of Immunology, College of Basic Medical Science, Dalian Medical University, Dalian 116044, Liaoning, China
2. Department of Rheumatology and Immunology, Peking University People's Hospital, Beijing 100044, China
CLC Number:
- R593.22
| 1 |
Ding Q , Hu W , Wang R , et al. Signaling pathways in rheumatoid arthritis: Implications for targeted therapy[J]. Signal Transduct Target Ther, 2023, 8 (1): 68.
doi: 10.1038/s41392-023-01331-9 |
| 2 |
McInnes IB , Schett G . The pathogenesis of rheumatoid arthritis[J]. N Engl J Med, 2011, 365 (23): 2205- 2219.
doi: 10.1056/NEJMra1004965 |
| 3 |
Chen W , Yu Y , Zheng SG , et al. Human gingival tissue-derived mesenchymal stem cells inhibit proliferation and invasion of rheumatoid fibroblast-like synoviocytes via the CD39/CD73 signaling pathway[J]. Rheumatol Autoimmun, 2023, 3, 90- 99.
doi: 10.1002/rai2.12075 |
| 4 | Wang D , Li Y , Liu Y , Shi G . The role of autoreactive T cell in the pathogenesis of rheumatoid arthritis and implications for T cell targeted vaccine therapy[J]. Minerva Med, 2015, 106 (3): 157- 167. |
| 5 |
Yamada H . The search for the pathogenic T cells in the joint of rheumatoid arthritis: Which T-cell subset drives autoimmune inflammation[J]. Int J Mol Sci, 2023, 24 (8): 6930.
doi: 10.3390/ijms24086930 |
| 6 |
Chemin K , Gerstner C , Malmström V . Effector functions of CD4+ T cells at the site of local autoimmune inflammation: Lessons from rheumatoid arthritis[J]. Front Immunol, 2019, 10, 353.
doi: 10.3389/fimmu.2019.00353 |
| 7 |
Yoshitomi H . Peripheral helper T cell responses in human diseases[J]. Front Immunol, 2022, 13, 946786.
doi: 10.3389/fimmu.2022.946786 |
| 8 |
Lucas C , Perdriger A , Amé P . Definition of B cell helper T cells in rheumatoid arthritis and their behavior during treatment[J]. Semin Arthritis Rheum, 2020, 50 (5): 867- 872.
doi: 10.1016/j.semarthrit.2020.06.021 |
| 9 |
Huang Y , Ba X , Han L , et al. T peripheral helper cells in autoimmune diseases: What do we know[J]. Front Immunol, 2023, 14, 1145573.
doi: 10.3389/fimmu.2023.1145573 |
| 10 | Sun S , Li H , Chen J , et al. Lactic acid: No longer an inert and end-product of glycolysis[J]. Physiology (Bethesda), 2017, 32 (6): 453- 463. |
| 11 |
Ye L , Jiang Y , Zhang M . Crosstalk between glucose metabolism, lactate production and immune response modulation[J]. Cytokine Growth Factor Rev, 2022, 68, 81- 92.
doi: 10.1016/j.cytogfr.2022.11.001 |
| 12 |
段姣妞, 张改连. 乳酸在类风湿关节炎发病机制及治疗中的研究进展[J]. 中华风湿病学杂志, 2022, 26 (12): 842- 845.
doi: 10.3760/cma.j.cn141217-20220323-00104 |
| 13 |
Haas R , Smith J , Rocher-Ros V , et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions[J]. PLoS Biol, 2015, 13 (7): e1002202.
doi: 10.1371/journal.pbio.1002202 |
| 14 |
Pucino V , Certo M , Bulusu V , et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4(+) T cell metabolic rewiring[J]. Cell Metab, 2019, 30 (6): 1055- 1074. e8.
doi: 10.1016/j.cmet.2019.10.004 |
| 15 |
Pucino V , Bombardieri M , Pitzalis C , et al. Lactate at the crossroads of metabolism, inflammation, and autoimmunity[J]. Eur J Immunol, 2017, 47 (1): 14- 21.
doi: 10.1002/eji.201646477 |
| 16 |
Chang X , Wei C . Glycolysis and rheumatoid arthritis[J]. Int J Rheum Dis, 2011, 14 (3): 217- 222.
doi: 10.1111/j.1756-185X.2011.01598.x |
| 17 | Wang Q , Asenso J , Xiao N , et al. Lactic acid regulation: A potential therapeutic option in rheumatoid arthritis[J]. J Immunol Res, 2022, 2022, 2280973. |
| 18 |
Rao DA , Gurish MF , Marshall JL , et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis[J]. Nature, 2017, 542 (7639): 110- 114.
doi: 10.1038/nature20810 |
| 19 |
Marks KE , Rao DA . T peripheral helper cells in autoimmune diseases[J]. Immunol Rev, 2022, 307 (1): 191- 202.
doi: 10.1111/imr.13069 |
| [1] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [2] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [3] | Xue ZOU,Xiao-juan BAI,Li-qing ZHANG. Effectiveness of tofacitinib combined with iguratimod in the treatment of difficult-to-treat moderate-to-severe rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1013-1021. |
| [4] | Qi WU,Yue-ming CAI,Juan HE,Wen-di HUANG,Qing-wen WANG. Correlation between dyslipidemia and rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 982-992. |
| [5] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Correlation analysis between body mass index and clinical characteristics of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 993-999. |
| [6] | Yin-ji JIN,Lin SUN,Jin-xia ZHAO,Xiang-yuan LIU. Significance of IgA isotype of anti-v-raf murine sarcoma viral oncogene homologue B1 antibody in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 631-635. |
| [7] | Wen-xin CAI,Shi-cheng LI,Yi-ming LIU,Ru-yu LIANG,Jing LI,Jian-ping GUO,Fan-lei HU,Xiao-lin SUN,Chun LI,Xu LIU,Hua YE,Li-zong DENG,Ru LI,Zhan-guo LI. A cross-sectional study on the clinical phenotypes of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1068-1073. |
| [8] | Fang CHENG,Shao-ying YANG,Xing-xing FANG,Xuan WANG,Fu-tao ZHAO. Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1074-1078. |
| [9] | Rui LIU,Jin-xia ZHAO,Liang YAN. Clinical characteristics of patients with rheumatoid arthritis complicated with venous thrombosis of lower extremities [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1079-1085. |
| [10] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Cross-sectional study on quality of life and disease activity of rheumatoid arthritis patients [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1086-1093. |
| [11] | GAO Chao,CHEN Li-hong,WANG Li,YAO Hong,HUANG Xiao-wei,JIA Yu-bo,LIU Tian. Validation of the Pollard’s classification criteria (2010) for rheumatoid arthritis patients with fibromyalgia [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 278-282. |
| [12] | LOU Xue,LIAO Li,LI Xing-jun,WANG Nan,LIU Shuang,CUI Ruo-mei,XU Jian. Methylation status and expression of TWEAK gene promoter region in peripheral blood of patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1020-1025. |
| [13] | ZHONG Hua,XU Li-ling,BAI Ming-xin,SU Yin. Effect of chemokines CXCL9 and CXCL10 on bone erosion in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1026-1031. |
| [14] | LUO Liang,HUO Wen-gang,ZHANG Qin,LI Chun. Clinical characteristics and risk factors of rheumatoid arthritis with ulcerative keratitis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1032-1036. |
| [15] | ZHANG Lu,HU Xiao-hong,CHEN Cheng,CAI Yue-ming,WANG Qing-wen,ZHAO Jin-xia. Analysis of cervical instability and clinical characteristics in treatment-naive rheumatoid arthritis patients [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1049-1054. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 115
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 225
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||