Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (6): 995-1000. doi: 10.19723/j.issn.1671-167X.2020.06.002
Previous Articles Next Articles
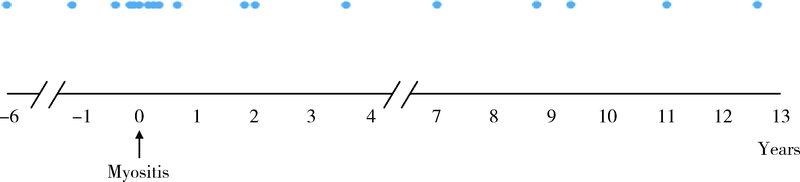
Clinical and immunological characteristics of myositis complicated with thromboembolism
Feng-yun-zhi ZHU1,Xiao-yan XING2,Xiao-fei TANG3,Yi-min LI1,Miao SHAO1,Xue-Wu ZHANG1,Yu-hui LI1,△( ),Xiao-lin SUN1,Jing HE1
),Xiao-lin SUN1,Jing HE1
- 1. Department of Rheumatology and Immunology, Peking University People’s Hospital, Beijing 100044, China
2. Department of Cardiac Electrophysiology, Peking University People’s Hospital, Beijing 100044, China
3. Department of Nephrology and Rheumatology, Aerospace Center Hospital, Beijing 100069, China
CLC Number:
- R593.2
| [1] |
Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups[J]. Arthritis Rheumatol, 2017,69(12):2271-2282.
doi: 10.1002/art.40320 pmid: 29106061 |
| [2] |
Ogdie A, Kay McGill N, Shin DB, et al. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: A general population-based cohort study[J]. Eur Heart J, 2018,39(39):3608-3614.
doi: 10.1093/eurheartj/ehx145 pmid: 28444172 |
| [3] |
Aviña-Zubieta JA, Vostretsova K, De Vera MA, et al. The risk of pulmonary embolism and deep venous thrombosis in systemic lupus erythematosus: A general population-based study[J]. Semin Arthritis Rheum, 2015,45(2):195-201.
doi: 10.1016/j.semarthrit.2015.05.008 pmid: 26364556 |
| [4] |
Carruthers EC, Choi HK, Sayre EC, et al. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: A general population-based study[J]. Ann Rheum Dis, 2016,75(1):110-116.
doi: 10.1136/annrheumdis-2014-205800 pmid: 25193998 |
| [5] | Antovic A, Notarnicola A, Svensson J, et al. Venous thromboembolic events in idiopathic inflammatory myopathy: Occurrence and relation to disease onset[J]. Arthritis Care Res (Hoboken), 2018,70(12):1849-1855. |
| [6] | Bohan A, Peter J B. Polymyositis and dermatomyositis (first of two parts)[J]. N Engl J Med, 1975,292(7):403-407. |
| [7] |
Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis siné myositis) as a distinctive subset within the idiopathic inflammatory dermatomyo-pathies spectrum of clinical illness?[J]. J Am Acad Dermatol, 2002,46(4):626-636.
pmid: 11907524 |
| [8] |
Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute[J]. Circulation, 2006,113(19):2363-2372.
pmid: 16702489 |
| [9] |
Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism[J]. J Thromb Thrombolysis, 2016,41(1):3-14.
pmid: 26780736 |
| [10] |
Holmqvist M, Ljung L, Askling J. Acute coronary syndrome in new-onset rheumatoid arthritis: A population-based nationwide cohort study of time trends in risks and excess risks[J]. Ann Rheum Dis, 2017,76(10):1642-1647.
doi: 10.1136/annrheumdis-2016-211066 pmid: 28710095 |
| [11] | Schoenfeld SR, Choi HK, Sayre EC, et al. Risk of pulmonary embolism and deep venous thrombosis in systemic sclerosis: A general population-based study[J]. Arthritis Care Res (Hoboken), 2016,68(2):246-253. |
| [12] | Zöller B, Li X, Sundquist J, et al. Risk of pulmonary embolism in patients with autoimmune disorders: A nationwide follow-up study from Sweden[J]. Lancet, 2012,379(9812):244-249. |
| [13] |
Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation[J]. Hamostaseologie, 2010,30(1):5-9.
pmid: 20162248 |
| [14] |
Choi HK, Rho YH, Zhu Y, et al. The risk of pulmonary embo-lism and deep vein thrombosis in rheumatoid arthritis: A UK population-based outpatient cohort study[J]. Ann Rheum Dis, 2013,72(7):1182-1187.
pmid: 22930596 |
| [15] |
Saghazadeh A, Rezaei N. Inflammation as a cause of venous thromboembolism[J]. Crit Rev Oncol Hematol, 2016,99:272-285.
doi: 10.1016/j.critrevonc.2016.01.007 pmid: 26811138 |
| [16] | Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis [J/OL]. Front Immunol, (2019-02-04)[2020-04-30]. doi: 10.3389/fimmu.2019.00085. |
| [17] | Ramagopalan SV, Wotton CJ, Handel AE, et al. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: Record-linkage study [J/OL]. BMC Med, (2011-01-10)[2020-04-30]. doi: 10.1186/1741-7015-9-1. |
| [1] | Zhengfang LI,Cainan LUO,Lijun WU,Xue WU,Xinyan MENG,Xiaomei CHEN,Yamei SHI,Yan ZHONG. Application value of anti-carbamylated protein antibody in the diagnosis of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 729-734. |
| [2] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [3] | Zhanhong LAI,Jiachen LI,Zelin YUN,Yonggang ZHANG,Hao ZHANG,Xiaoyan XING,Miao SHAO,Yuebo JIN,Naidi WANG,Yimin LI,Yuhui LI,Zhanguo LI. A unicenter real-world study of the correlation factors for complete clinical response in idiopathic inflammatory myopathies [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 284-292. |
| [4] | Yan-hong MENG,Yi-fan CHEN,Pei-ru ZHOU. Clinical and immunological features of primary Sjögren's syndrome patients with positive anti-centromere protein B antibody [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1088-1096. |
| [5] | Qing PENG,Jia-jun LIU,Yan LIU,Hua SHANG,Guo TANG,Ya-xin HAN,Li LONG. Application of Padua prediction score and serum albumin level in evaluating venous thromboembolism in rheumatic inpatients [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 625-630. |
| [6] | Lu ZHANG,Cheng CHEN,Mei-ting WENG,Ai-ping ZHENG,Mei-ling SU,Qing-wen WANG,Yue-ming CAI. Characteristics of serum autoantibodies in patients with lupus nephritis and tubulointerstitial damage [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1094-1098. |
| [7] | Xiao-yan XING,Jun-xiao ZHANG,Feng-yun-zhi ZHU,Yi-fan WANG,Xin-yao ZHOU,Yu-hui LI. Clinical analysis of 5 cases of dermatomyositis complicated with macrophage activation syndrome [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1214-1218. |
| [8] | Yi-cen YING,Qi TANG,Kai-wei YANG,Yue MI,Yu FAN,Wei YU,Yi SONG,Zhi-song HE,Li-qun ZHOU,Xue-song LI. Clinical features of immune checkpoint inhibitor-related myositis in patients with urological cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 644-651. |
| [9] | ZHANG Pu-li,YANG Hong-xia,ZHANG Li-ning,GE Yong-peng,PENG Qing-lin,WANG Guo-chun,LU Xin. Value of serum YKL-40 in the diagnosis of anti-MDA5-positive patients with dermatomyositis complicated with severe pulmonary injury [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1055-1060. |
| [10] | LUO Lan,XING Xiao-yan,XIAO Yun-shu,CHEN Ke-yan,ZHU Feng-yun-zhi,ZHANG Xue-wu,LI Yu-hui. Clinical and immunological characteristics of patients with anti-synthetase syndrome complicated with cardiac involvement [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1078-1082. |
| [11] | XIAO Yun-shu,ZHU Feng-yun-zhi,LUO Lan,XING Xiao-yan,LI Yu-hui,ZHANG Xue-wu,SHEN Dan-hua. Clinical and immunological characteristics of 88 cases of overlap myositis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1088-1093. |
| [12] | YI Wen-xia,WEI Cui-jie,WU Ye,BAO Xin-hua,XIONG Hui,CHANG Xing-zhi. Long-term rituximab treatment of refractory idiopathic inflammatory myopathy: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1191-1195. |
| [13] | Yu-zhou GAN,Yu-hui LI,Li-hua ZHANG,Lin MA,Wen-wen HE,Yue-bo JIN,Yuan AN,Zhan-guo LI,Hua YE. Comparison of clinical and immunological features between clinically amyopathic dermatomyositis and typical dermatomyositis [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1001-1008. |
| [14] | Jing ZHAO,Feng SUN,Yun LI,Xiao-zhen ZHAO,Dan XU,Ying-ni LI,Yu-hui LI,Xiao-lin SUN. Significance of anti-tubulin-α-1C autoantibody in systemic sclerosis [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1009-1013. |
| [15] | Yi-ming ZHENG,Hong-jun HAO,Yi-lin LIU,Jing GUO,Ya-wen ZHAO,Wei ZHANG,Yun YUAN. Correlation study on anti-Ro52 antibodies frequently co-occur with other myositis-specific and myositis-associated autoantibodies [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1088-1092. |
|
||