Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (6): 1151-1157. doi: 10.19723/j.issn.1671-167X.2022.06.015
Previous Articles Next Articles
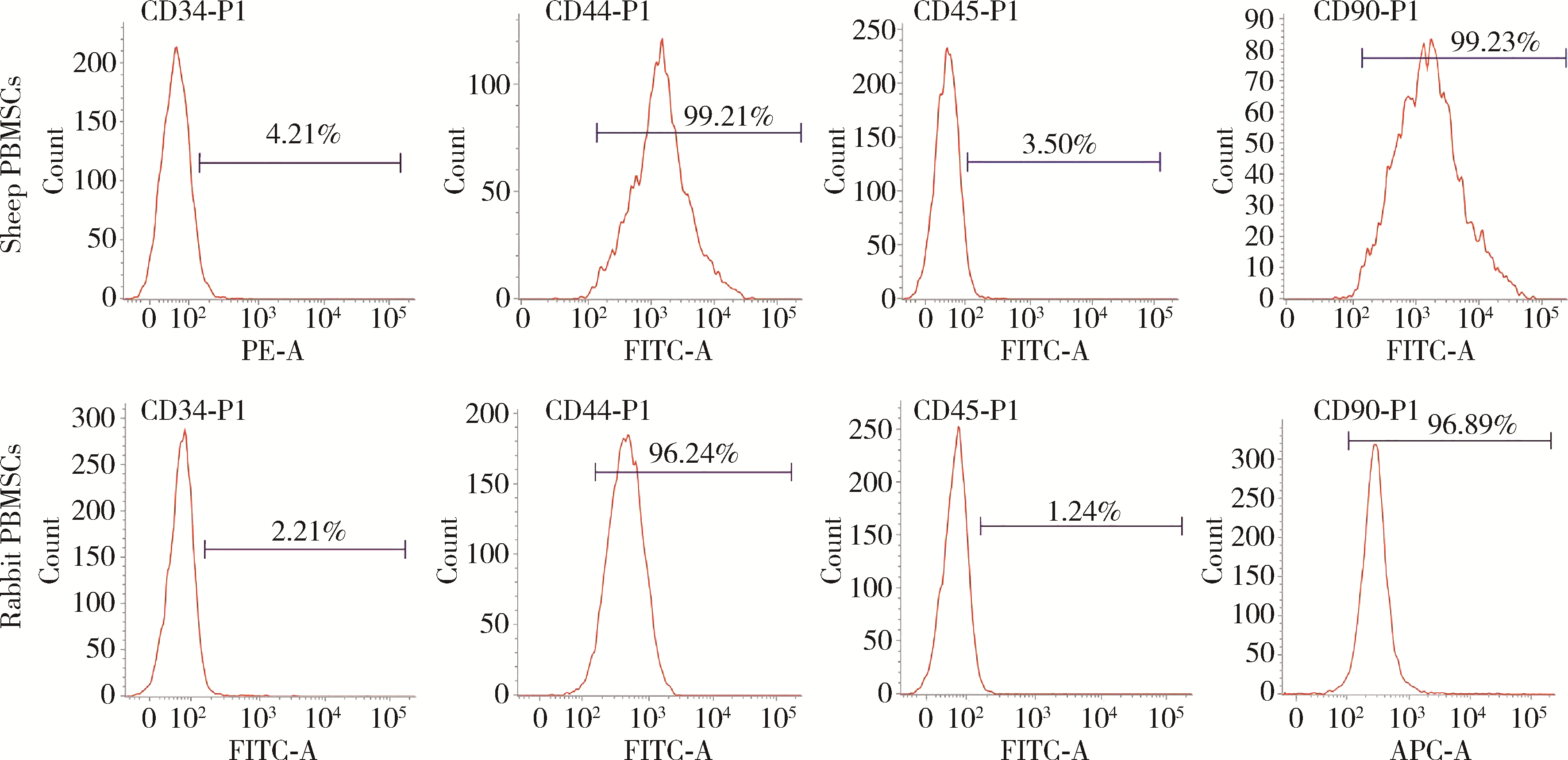
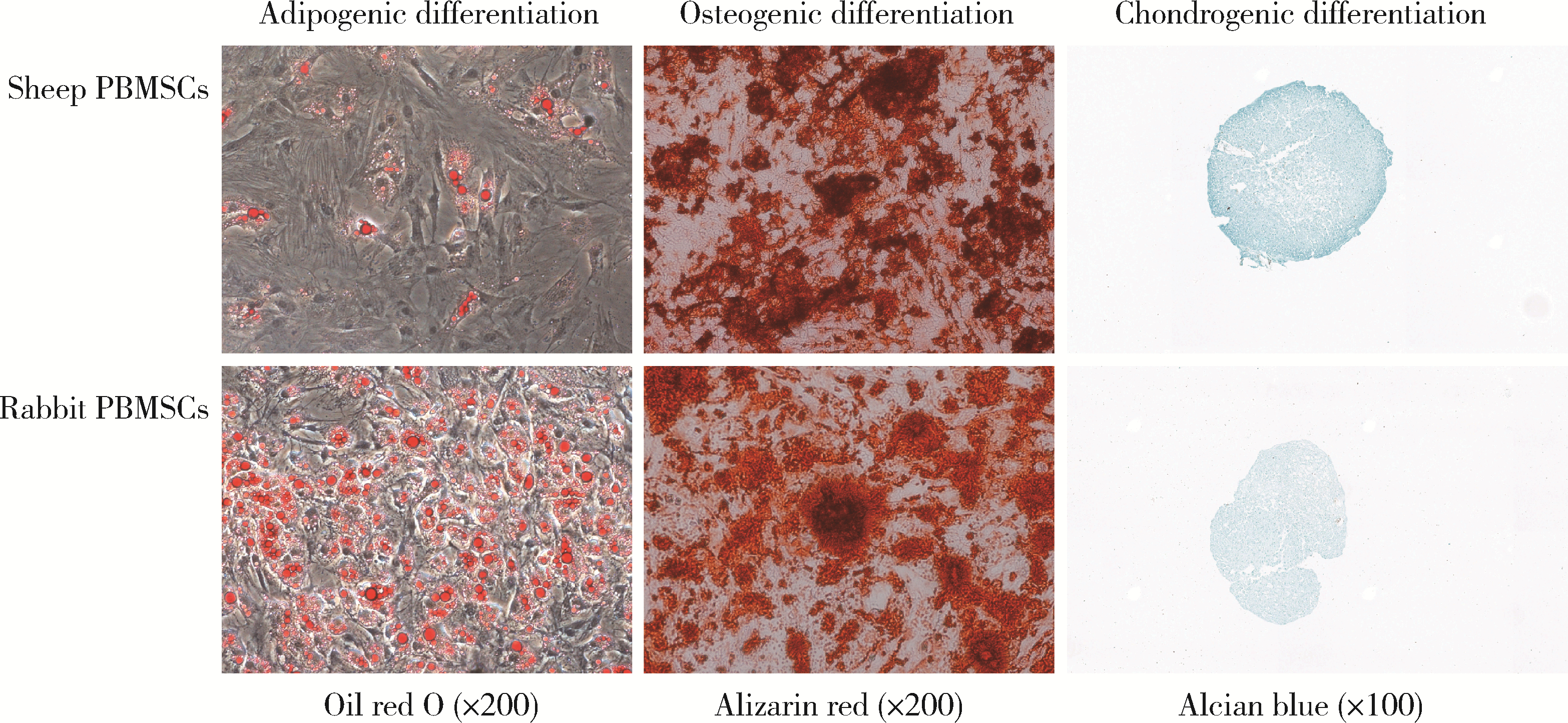
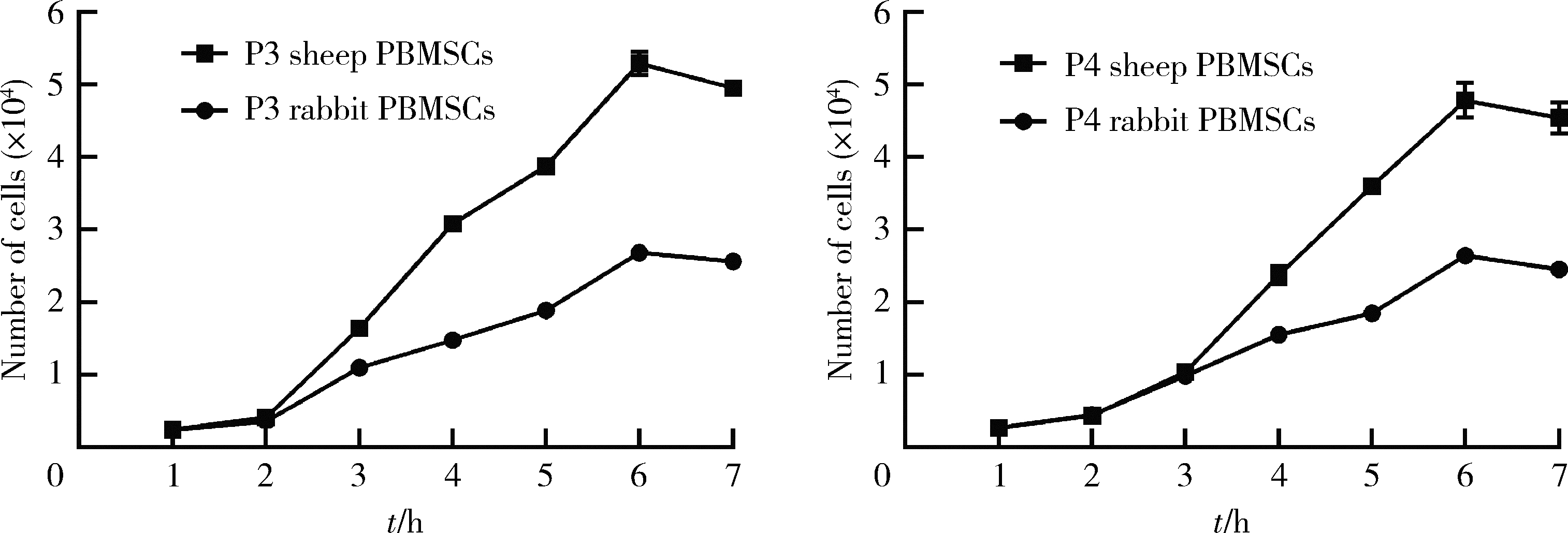
Biological characteristics of sheep peripheral blood mesenchymal stem cell
Chao HAN1,Zhu-xing ZHOU2,You-rong CHEN2,Zi-hui DONG1,Jia-kuo YU2,*( )
)
- 1. Weifang Medical University, Weifang 261053, Shandong, China
2. Department of Sports Medicine, Beijing 100191, China
CLC Number:
- R34
| 1 |
Luz-Crawford P , Hernandez J , Djouad F , et al. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer[J]. Stem Cell Res Ther, 2019, 10 (1): 232.
doi: 10.1186/s13287-019-1307-9 |
| 2 |
Shahror RA , Ali AAA , Wu CC , et al. Enhanced homing of mesenchymal stem cells overexpressing fibroblast growth factor 21 to injury site in a mouse model of traumatic brain injury[J]. Int J Mol Sci, 2019, 20 (11): e2624.
doi: 10.3390/ijms20112624 |
| 3 |
de Windt TS , Vonk LA , Slaper-Cortenbach I , et al. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: a first-in-man trial in 35 patients[J]. Stem Cells, 2017, 35 (8): 1984- 1993.
doi: 10.1002/stem.2657 |
| 4 |
Al-Najar M , Khalil H , Al-Ajlouni J , et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase Ⅰ/Ⅱ study[J]. J Orthop Surg Res, 2017, 12 (1): 190.
doi: 10.1186/s13018-017-0689-6 |
| 5 |
Park YB , Ha CW , Lee CH , et al. Restoration of a large osteochondral defect of the knee using a composite of umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel: A case report with a 5-year follow-up[J]. BMC Musculoske-let Disord, 2017, 18 (1): 59.
doi: 10.1186/s12891-017-1422-7 |
| 6 |
Gobbi A , Whyte GP . One-stage cartilage repair using a hyaluronic acid-based scaffold with activated bone marrow-derived mesenchymal stem cells compared with microfracture: Five-year follow-up[J]. Am J Sports Med, 2016, 44 (11): 2846- 2854.
doi: 10.1177/0363546516656179 |
| 7 | Chen XB , Wang L , Hou JY , et al. Study on the dynamic biological characteristics of human bone marrow mesenchymal stem cell senescence[J]. Stem Cells Int, 2019, 2019, 9271595. |
| 8 |
Hu PF , Pu YB , Li XY , et al. Isolation, in vitro culture and identification of a new type of mesenchymal stem cell derived from fetal bovine lung tissues[J]. Mol Med Rep, 2015, 12 (3): 3331- 3338.
doi: 10.3892/mmr.2015.3854 |
| 9 | Zheng PF , Hu XY , Lou Y , et al. A rabbit model of osteochondral regeneration using three-dimensional printed polycaprolactone-hydroxyapatite scaffolds coated with umbilical cord blood mesenchymal stem cells and chondrocytes[J]. Med Sci Monit, 2019, 25, 7361- 7369. |
| 10 |
Zhan XS , El-Ashram S , Luo DZ , et al. A comparative study of biological characteristics and transcriptome profiles of mesenchymal stem cells from different canine tissues[J]. Int J Mol Sci, 2019, 20 (6): e1485.
doi: 10.3390/ijms20061485 |
| 11 |
Lee Y , Chan Y , Hsieh S , et al. Comparing the osteogenic potentials and bone regeneration capacities of bone marrow and dental pulp mesenchymal stem cells in a rabbit calvarial bone defect mo-del[J]. Int J Mol Sci, 2019, 20 (20): e5015.
doi: 10.3390/ijms20205015 |
| 12 |
Bharti D , Shivakumar SB , Park JK , et al. Comparative analysis of human Wharton's jelly mesenchymal stem cells derived from different parts of the same umbilical cord[J]. Cell Tissue Res, 2018, 372 (1): 51- 65.
doi: 10.1007/s00441-017-2699-4 |
| 13 |
Heo JS , Choi Y , Kim HS , et al. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue[J]. Int J Mol Med, 2016, 37 (1): 115- 125.
doi: 10.3892/ijmm.2015.2413 |
| 14 |
Li ZL , Liu CX , Xie ZH , et al. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation[J]. PLoS One, 2011, 6 (6): e20526.
doi: 10.1371/journal.pone.0020526 |
| 15 |
Zhu XS , He BX , Zhou XN , et al. Comparison of the effects of human adipose and bone marrow mesenchymal stem cells on T lymphocytes[J]. Cell Biol Int, 2013, 37 (1): 11- 18.
doi: 10.1002/cbin.10002 |
| 16 |
Fu WL , Zhou CY , Yu JK . A new source of mesenchymal stem cells for articular cartilage repair: MSCs derived from mobilized peripheral blood share similar biological characteristics in vitro and chondrogenesis in vivo as MSCs from bone marrow in a rabbit model[J]. Am J Sports Med, 2014, 42 (3): 592- 601.
doi: 10.1177/0363546513512778 |
| 17 |
Wang SJ , Yin MH , Jiang DH , et al. The chondrogenic potential of progenitor cells derived from peripheral blood: A systematic review[J]. Stem Cells Dev, 2016, 25 (16): 1195- 1207.
doi: 10.1089/scd.2016.0055 |
| 18 |
Sasaki T , Akagi R , Akatsu Y , et al. The effect of systemic administration of G-CSF on a full-thickness cartilage defect in a rabbit model MSC proliferation as presumed mechanism: G-CSF for cartilage repair[J]. Bone Joint Res, 2017, 6 (3): 123- 131.
doi: 10.1302/2046-3758.63.BJR-2016-0083 |
| 19 |
Delgaudine M , Lambermont B , Lancellotti P , et al. Effects of granulocyte-colony-stimulating factor on progenitor cell mobilization and heart perfusion and function in normal mice[J]. Cytotherapy, 2011, 13 (2): 237- 247.
doi: 10.3109/14653249.2010.491820 |
| 20 |
Fu QH , Tang NN , Zhang QN , et al. Preclinical study of cell therapy for osteonecrosis of the femoral head with allogenic peri-pheral blood-derived mesenchymal stem cells[J]. Yonsei Med J, 2016, 57 (4): 1006- 1015.
doi: 10.3349/ymj.2016.57.4.1006 |
| 21 |
Music E , Futrega K , Doran MR . Sheep as a model for evaluating mesenchymal stem/stromal cell (MSC)-based chondral defect repair[J]. Osteoarthritis Cartilage, 2018, 26 (6): 730- 740.
doi: 10.1016/j.joca.2018.03.006 |
| 22 |
Landa-Solis C , Granados-Montiel J , Olivos-Meza A , et al. Cryopreserved CD90+cells obtained from mobilized peripheral blood in sheep: A new source of mesenchymal stem cells for preclinical applications[J]. Cell Tissue Bank, 2016, 17 (1): 137- 145.
doi: 10.1007/s10561-015-9526-5 |
| 23 |
Martínez-Lorenzo MJ , Royo-Cañas M , Alegre-Aguarón E , et al. Phenotype and chondrogenic differentiation of mesenchymal cells from adipose tissue of different species[J]. J Orthop Res, 2009, 27 (11): 1499- 1507.
doi: 10.1002/jor.20898 |
| 24 |
Dhar M , Neilsen N , Beatty K , et al. Equine peripheral blood-derived mesenchymal stem cells: Isolation, identification, trilineage differentiation and effect of hyperbaric oxygen treatment[J]. Equine Vet J, 2012, 44 (5): 600- 605.
doi: 10.1111/j.2042-3306.2011.00536.x |
| 25 | 孙良, 栾保华, 李中华, 等. 兔外周血间充质干细胞的体外分离培养及诱导成骨[J]. 中国组织工程研究与临床康复, 2009, 13 (27): 5291- 5295. |
| 26 |
Liu LZ , Yu Q , Lin J , et al. Hypoxia-inducible factor-1alpha is essential for hypoxia-induced mesenchymal stem cell mobilization into the peripheral blood[J]. Stem Cells Dev, 2011, 20 (11): 1961- 1971.
doi: 10.1089/scd.2010.0453 |
| 27 |
Salazar TE , Richardson MR , Beli E , et al. Electroacupuncture promotes central nervous system-dependent release of mesenchymal stem cells[J]. Stem cells (Dayton, Ohio), 2017, 35 (5): 1303- 1315.
doi: 10.1002/stem.2613 |
| 28 |
张继英, 刘刚, 马栋, 等. Sprague-Dawley大鼠外周血来源间充质干细胞的动员、分离培养及鉴定[J]. 中国运动医学杂志, 2012, 31 (9): 811- 816.
doi: 10.3969/j.issn.1000-6710.2012.09.010 |
| 29 |
Ri M , Matsue K , Sunami K , et al. Effcacy and safety of plerixafor for the mobilization collection of peripheral hematopoietic stem cells for autologous transplantation in Japanese patients with multiple myeloma[J]. Int J Hematol, 2017, 106 (4): 562- 572.
doi: 10.1007/s12185-017-2255-8 |
| 30 | 刘岐焕, 陈龙, 程范军, 等. 鼠尾胶在小鼠外周血间充质干细胞分离培养中的作用[J]. 中国组织工程研究与临床康复, 2008, 12 (21): 4017- 4020. |
| 31 | 王东来, 冯建刚. 干细胞因子和粒细胞集落刺激因子联合动员小鼠外周血培养间充质干细胞的生物学特性[J]. 中国组织工程研究与临床康复, 2008, 12 (16): 3105- 3109. |
| 32 | Daems R , van Hecke L , Schwarzkopf I , et al. A feasibility study on the use of equine chondrogenic induced mesenchymal stem cells as a treatment for natural occurring osteoarthritis in dogs[J]. Stem Cells Int, 2019, 2019, 4587594. |
| [1] | Zhi-wei LIU,Peng LIU,Fan-xing MENG,Tian-shui LI,Ying WANG,Jia-qi GAO,Zuo-yi ZHOU,Cong WANG,Bin ZHAO. Regulative effects of endogenous sulfur dioxide on oxidant stress in myocardium of rat with sepsis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 582-586. |
| [2] | 伟岗 方,新霞 田,云涛 解. [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 822-828. |
| [3] | Jing ZHANG,Jia-gui SONG,Zhen-bin WANG,Yu-qing GONG,Tian-zhuo WANG,Jin-yu ZHOU,Jun ZHAN,Hong-quan ZHANG. Kindlin-2 regulates endometrium development via mTOR and Hippo signaling pathways in mice [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 846-852. |
| [4] | Tian-yu CAI,Zhen-peng ZHU,Chun-ru XU,Xing JI,Tong-de LV,Zhen-ke GUO,Jian LIN. Expression and significance of fibroblast growth factor receptor 2 in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 628-635. |
| [5] | SHUAI Ting,LIU Juan,GUO Yan-yan,JIN Chan-yuan. Knockdown of long non-coding RNA MIR4697 host gene inhibits adipogenic differentiation in bone marrow mesenchymal stem cells [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 320-326. |
| [6] | TIAN Ai-ju, LI Zi-jian. 14-3-3/HIP-55 complex increases the stability of HIP-55 [J]. Journal of Peking University(Health Sciences), 2015, 47(6): 893-897. |
|
||