Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (6): 1013-1021. doi: 10.19723/j.issn.1671-167X.2023.06.009
Previous Articles Next Articles
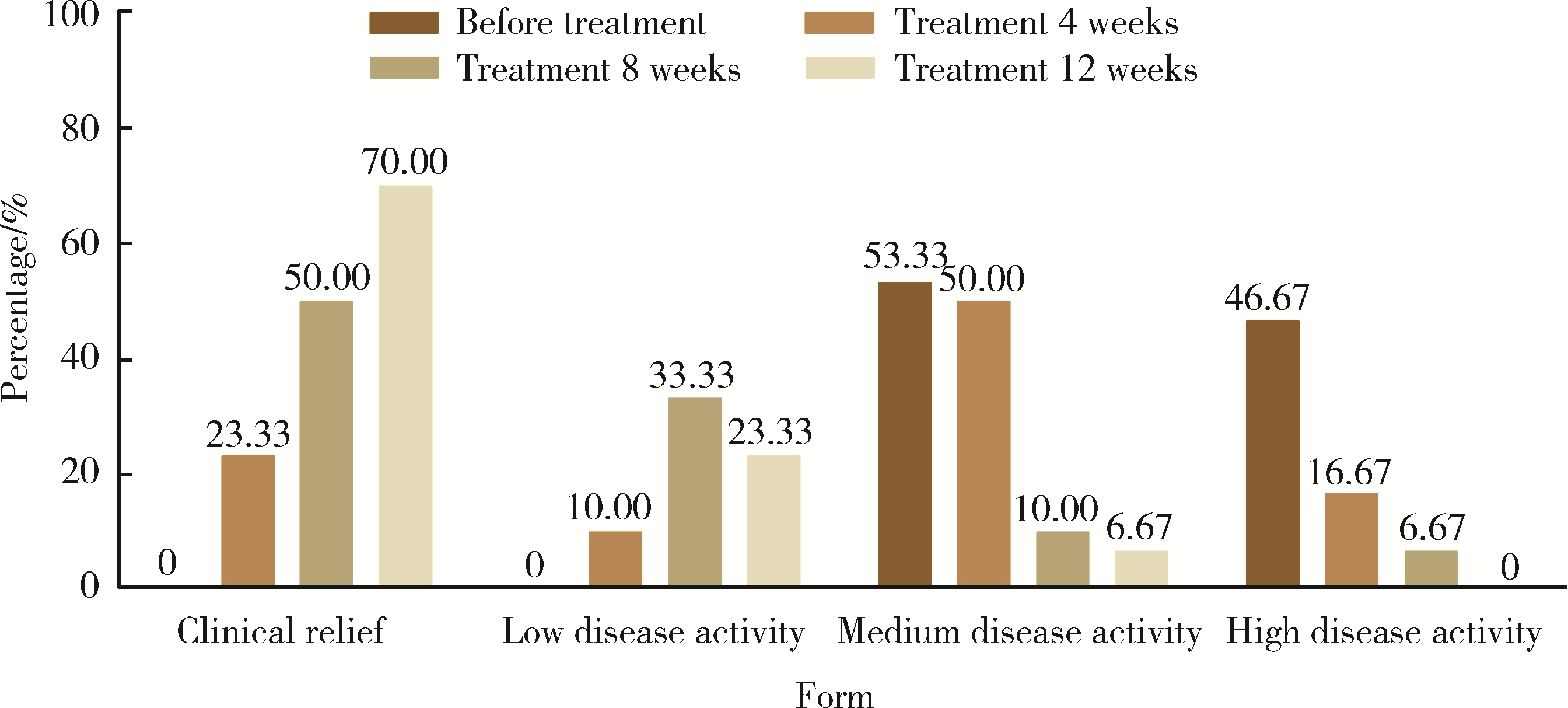
Effectiveness of tofacitinib combined with iguratimod in the treatment of difficult-to-treat moderate-to-severe rheumatoid arthritis
Xue ZOU1,2,Xiao-juan BAI1,Li-qing ZHANG1,*( )
)
- 1. Department of Rheumatology and Immunology, Fenyang Hospital Affiliated to Shanxi Medical University, Shanxi Province Fenyang Hospital, Fenyang 032200, Shanxi, China
2. Department of Gastroenterology, Suzhou Yongding Hospital, Suzhou 215100, Jiangsu, China
CLC Number:
- R593.22
| 1 |
Smolen JS , Landewé RBM , Bijlsma JWJ , et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying anti-rheumatic drugs: 2019 update[J]. Ann Rheum Dis, 2020, 79 (6): 685- 699.
doi: 10.1136/annrheumdis-2019-216655 |
| 2 | de Hair MJH , Jacobs JWG , Schoneveld JLM , et al. Difficult-to-treat rheumatoid arthritis: An area of unmet clinical need[J]. Rheumatology (Oxford), 2018, 57 (7): 1135- 1144. |
| 3 |
Buch MH . Defining refractory rheumatoid arthritis[J]. Ann Rheum Dis, 2018, 77 (7): 966- 969.
doi: 10.1136/annrheumdis-2017-212862 |
| 4 |
Smolen JS , Aletaha D , Mcinnes IB . Rheumatoid arthritis[J]. Lancet, 2016, 388 (10055): 2023- 2038.
doi: 10.1016/S0140-6736(16)30173-8 |
| 5 |
Aletaha D , Neogi T , Silman AJ , et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative initiative[J]. Arthritis Rheum, 2010, 62 (9): 2569- 2581.
doi: 10.1002/art.27584 |
| 6 |
Wu D , Luo Y , Li T , et al. Systemic complications of rheumatoid arthritis: Focus on pathogenesis and treatment[J]. Front Immunol, 2022, 13, 1051082.
doi: 10.3389/fimmu.2022.1051082 |
| 7 |
Sun X , Li R , Cai Y , et al. Clinical remission of rheumatoid arthritis in a multi center real-world study in Asia-Pacific region[J]. Lancet Reg Health West Pac, 2021, 15, 100240.
doi: 10.1016/j.lanwpc.2021.100240 |
| 8 | 李宏超, 徐丽玲, 苏茵. 难治性类风湿关节炎诊治探讨[J]. 中华风湿病学杂志, 2019, 23 (10): 689- 693. |
| 9 |
Nagy G , Roodenrijs NMT , Welsing PM , et al. EULAR definition of difficult-to-treat rheumatoid arthritis[J]. Ann Rheum Dis, 2021, 80 (1): 31- 35.
doi: 10.1136/annrheumdis-2020-217344 |
| 10 |
Xie S , Li S , Tian J , et al. Iguratimod as a new drug for rheumatoid arthritis: Current landscape[J]. Front Pharmacol, 2020, 11, 73.
doi: 10.3389/fphar.2020.00073 |
| 11 | Xu Y , Zhu Q , Song J , et al. Regulatory effect of iguratimod on the balance of Th subsets and inhibition of inflammatory cytokines in patients with rheumatoid arthritis[J]. Mediators Inflamm, 2015, 2015, 356040. |
| 12 | Wen L , Jiang W , Zhou M , et al. Effect of combined application of iguratimod in the treatment of active rheumatoid arthritis on bone metabolism, Th17 cells and Treg cells[J]. Am J Transl Res, 2021, 13 (3): 1676- 1684. |
| 13 |
Liu S , Song LP , Li RB , et al. Iguratimod promotes transformation of mononuclear macrophages in elderly patients with rheumatoid arthritis by nuclear factor-κB pathway[J]. World J Clin Cases, 2021, 9 (10): 2181- 2191.
doi: 10.12998/wjcc.v9.i10.2181 |
| 14 |
Li CH , Ma ZZ , Jian LL , et al. Iguratimod inhibits osteoclastoge-nesis by modulating the RANKL and TNF-α signaling pathways[J]. Int Immunopharmacol, 2021, 90, 107219.
doi: 10.1016/j.intimp.2020.107219 |
| 15 |
Kondo N , Kuroda T , Kobayashi D . Cytokine networks in the pa-thogenesis of rheumatoid arthritis[J]. Int J Mol Sci, 2021, 22 (20): 10922.
doi: 10.3390/ijms222010922 |
| 16 | Malemud CJ . The role of the JAK/STAT signal pathway in rheumatoid arthritis[J]. Ther Adv Musculoskelet Dis, 2018, 10 (5/6): 117- 127. |
| 17 | 疏金玲, 张玲玲, 魏伟. 酪氨酸激酶抑制剂治疗类风湿关节炎研究进展[J]. 中国药理学与毒理学杂志, 2020, 34 (9): 713- 720. |
| 18 |
Puigdevall L , Michiels C , Stewardson C , et al. JAK/STAT: Why choose a classical or an alternative pathway when you can have both?[J]. J Cell Mol Med, 2022, 26 (7): 1865- 1875.
doi: 10.1111/jcmm.17168 |
| 19 | 戴冰冰, 刘佳丽, 李宁宁, 等. 托法替布治疗难治性中重度类风湿关节炎的疗效及安全性[J]. 实用临床医药杂志, 2022, 26 (11): 122- 126. |
| 20 |
Zheng N , Guo C , Wu R . Iguratimod is effective in refractory rheumatoid arthritis patients with inadequate response to metho-trexate-cyclosporin A-hydroxychloroquine-prednisone[J]. Scand J Rheumatol, 2018, 47 (5): 422- 424.
doi: 10.1080/03009742.2017.1376109 |
| 21 |
Mizutani S , Kodera H , Sato Y , et al. Clinical effectiveness of iguratimod based on real-world data of patients with rheumatoid arthritis[J]. Clin Rheumatol, 2021, 40 (1): 123- 132.
doi: 10.1007/s10067-020-05208-y |
| 22 | Inoue A , Nozaki Y , Hirooka Y , et al. The effectiveness and retention rate of iguratimod in Japanese rheumatoid arthritis patients with/without methotrexate in daily medical care[J]. Life (Basel), 2020, 10 (11): 261. |
| 23 | Ouyang D , Ma YZ , Zou J , et al. Effectiveness and safety of iguratimod monotherapy or combined with methotrexate in treating rheumatoid arthritis: A aystematic review and meta-analysis[J]. Front Pharmacol, 2022, 13, 911810. |
| 24 | Smolen JS , Landewe R , Breedveld FC , et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update[J]. Ann Rheum Dis, 2014, 73 (3): 492- 509. |
| 25 | Angelini J , Talotta R , Roncato R , et al. JAK-inhibitors for the treatment of rheumatoid arthritis: A focus on the present and an outlook on the future[J]. Biomolecules, 2020, 10 (7): 1002. |
| 26 | 张春燕, 范小冬, 秦元, 等. JAK抑制剂托法替布治疗类风湿性关节炎效果的Meta分析[J]. 第三军医大学学报, 2018, 40 (6): 543- 550. |
| 27 | Sands BE , Taub PR , Armuzzi A , et al. Tofacitinib treatment is associated with modest and reversible increases in serum lipids in patients with ulcerative colitis[J]. Clin Gastroenterol Hepatol, 2020, 18 (1): 123- 132.e3. |
| 28 | Taylor PC , Kremer JM , Emery P , et al. Lipid profile and effect of statin treatment in pooled phase Ⅱ and phase Ⅲ baricitinib studies[J]. Ann Rheum Dis, 2018, 77 (7): 988- 995. |
| [1] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [2] | Zuoxiang LIU,Xiaowei CHEN,Houyu ZHAO,Siyan ZHAN,Feng SUN. Cardiovascular safety of sitagliptin added to metformin in real world patients with type 2 diabetes [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 424-430. |
| [3] | Huina HUANG,Jing ZHAO,Xiangge ZHAO,Ziran BAI,Xia LI,Guan WANG. Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 519-525. |
| [4] | Shan HE,Xin CHEN,Qi CHENG,Lingjiang ZHU,Peiyu ZHANG,Shuting TONG,Jing XUE,Yan DU. Tofacitinib inhibits the transformation of lung fibroblasts into myofibroblasts through JAK/STAT3 pathway [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 505-511. |
| [5] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [6] | Wenjing LI,Baozhou ZHANG,Heng LI,Liangpeng LAI,Hui DU,Ning SUN,Xiaofeng GONG,Ying LI,Yan WANG,Yong WU. Tibiotalocalcaneal arthrodesis for end-stage ankle and hindfoot arthropathy: Short- and mid-term clinical outcomes [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 299-306. |
| [7] | Qi WU,Yue-ming CAI,Juan HE,Wen-di HUANG,Qing-wen WANG. Correlation between dyslipidemia and rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 982-992. |
| [8] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Correlation analysis between body mass index and clinical characteristics of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 993-999. |
| [9] | Min QIU,You-long ZONG,Bin-shuai WANG,Bin YANG,Chu-xiao XU,Zheng-hui SUN,Min LU,Lei ZHAO,Jian LU,Cheng LIU,Xiao-jun TIAN,Lu-lin MA. Treatment outcome of laparoscopic partial nephrectomy in patients with renal tumors of moderate to high complexity [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 833-837. |
| [10] | Yin-ji JIN,Lin SUN,Jin-xia ZHAO,Xiang-yuan LIU. Significance of IgA isotype of anti-v-raf murine sarcoma viral oncogene homologue B1 antibody in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 631-635. |
| [11] | Lei WANG,Tian-dong HAN,Wei-xing JIANG,Jun LI,Dao-xin ZHANG,Ye TIAN. Comparison of safety and effectiveness of active migration technique and in situ lithotripsy technique in the treatment of 1-2 cm upper ureteral calculi by flexible ure-teroscopy [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 553-557. |
| [12] | Wen-xin CAI,Shi-cheng LI,Yi-ming LIU,Ru-yu LIANG,Jing LI,Jian-ping GUO,Fan-lei HU,Xiao-lin SUN,Chun LI,Xu LIU,Hua YE,Li-zong DENG,Ru LI,Zhan-guo LI. A cross-sectional study on the clinical phenotypes of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1068-1073. |
| [13] | Fang CHENG,Shao-ying YANG,Xing-xing FANG,Xuan WANG,Fu-tao ZHAO. Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1074-1078. |
| [14] | Rui LIU,Jin-xia ZHAO,Liang YAN. Clinical characteristics of patients with rheumatoid arthritis complicated with venous thrombosis of lower extremities [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1079-1085. |
| [15] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Cross-sectional study on quality of life and disease activity of rheumatoid arthritis patients [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1086-1093. |
|
||