Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (2): 213-222. doi: 10.19723/j.issn.1671-167X.2024.02.003
Previous Articles Next Articles
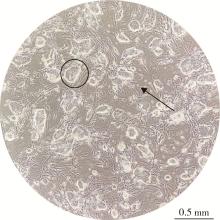
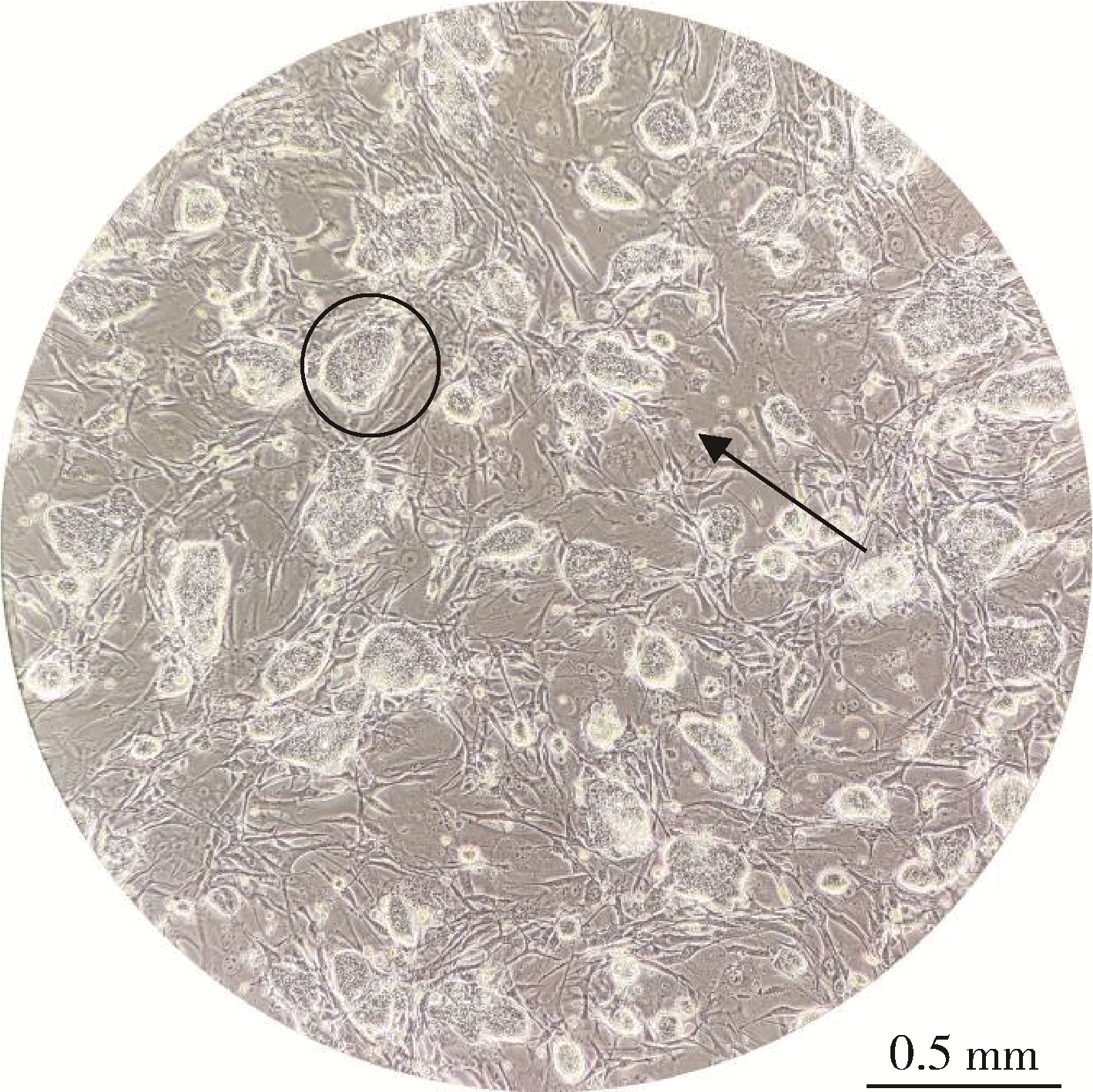
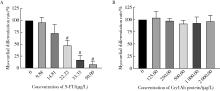
Developmental toxicity of Cry1Ab protein in the embryonic stem-cell model
Yuanzhi JIAN1,Fei WANG1,Ning YIN1,Ruoyu ZHOU1,Junbo WANG1,2,*( )
)
- 1. Department of Nutrition and Food Hygiene, School of Public Health, Peking University, Beijing 100191, China
2. Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety, Beijing 100191, China
CLC Number:
- R155.5
| 1 |
SchnepfE,CrickmoreN,Van RieJ,et al.Bacillus thuringiensis and its pesticidal crystal proteins[J].Microbiol Mol Biol Rev,1998,62(3):775-806.
doi: 10.1128/MMBR.62.3.775-806.1998 |
| 2 |
Rubio-InfanteN,Moreno-FierrosL.An overview of the safety and biological effects of Bacillus thuringiensis Cry toxins in mammals[J].J Appl Toxicol,2016,36(5):630-648.
doi: 10.1002/jat.3252 |
| 3 | ÁlvarezF,MesséanA,StreisslF.Assessment of the 2019 post-market environmental monitoring report on the cultivation of genetically modified maize MON810 in the EU[J].EFSA J,2021,19(7):e06683. |
| 4 | KochMS,WardJM,LevineSL,et al.The food and environmental safety of Bt crops[J].Front Plant Sci,2015,6,283. |
| 5 | VieiraL,HissaDC,SouzaT,et al.Assessing the effects of an acute exposure to worst-case concentration of Cry proteins on zebrafish using the embryotoxicity test and proteomics analysis[J].Chemosphere,2021,264(Pt 2):128538. |
| 6 |
BøhnT,RoverCM,SemenchukPR.Daphnia magna negatively affected by chronic exposure to purified Cry-toxins[J].Food Chem Toxicol,2016,91,130-140.
doi: 10.1016/j.fct.2016.03.009 |
| 7 |
AlvesRDC,FerreiraCGM,Ferreira de MeloIM,et al.Renal and hepatic changes in the offspring of rats that received biological insecticides during pregnancy and lactation[J].Acta Histochem,2021,123(8):151799.
doi: 10.1016/j.acthis.2021.151799 |
| 8 |
MesnageR,ClairE,GressS,et al.Cytotoxicity on human cells of Cry1Ab and Cry1Ac Bt insecticidal toxins alone or with a glyphosate-based herbicide[J].J Appl Toxicol,2013,33(7):695-699.
doi: 10.1002/jat.2712 |
| 9 |
Mendoza-AlmanzaG,Rocha-ZavaletaL,Aguilar-ZacaríasC,et al.Cry1A Proteins are cytotoxic to HeLa but not to SiHa cervical cancer cells[J].Curr Pharm Biotechnol,2019,20(12):1018-1027.
doi: 10.2174/1389201020666190802114739 |
| 10 |
SoberónM,PortugalL,Garcia-GómezBI,et al.Cell lines as models for the study of Cry toxins from Bacillus thuringiensis[J].Insect Biochem Mol Biol,2018,93,66-78.
doi: 10.1016/j.ibmb.2017.12.008 |
| 11 |
GenschowE,SpielmannH,ScholzG,et al.Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests[J].Altern Lab Anim,2004,32(3):209-244.
doi: 10.1177/026119290403200305 |
| 12 |
LiuH,RenC,LiuW,et al.Embryotoxicity estimation of commonly used compounds with embryonic stem cell test[J].Mol Med Rep,2017,16(1):263-271.
doi: 10.3892/mmr.2017.6552 |
| 13 | zur NiedenN I,RufL J,KempkaG,et al.Molecular markers in embryonic stem cells[J].Toxicol In Vitro,2001,15(4/5):455-461. |
| 14 | SpielmannH,PohlI,DöringB,et al.The embryonic stem cell test, an in vitro embryotoxicity test using two permanent mouse cell lines: 3T3 fibroblasts and embryonic stem cells[J].In Vitr Mol Toxicol,1997,10(1):119-127. |
| 15 | ECVAM. DB-ALM protocol n° 113: Embryonic stem cell test (EST)[G]. EURL ECVAM, 2010. https://jeodpp.jrc.ec.europa.eu/ftp/jrc-opendata/EURL-ECVAM/datasets/DBALM/LATEST/online/DBALM_docs/113_P_Embryonic%20Stem%20Cell%20Test.pdf. |
| 16 |
PesceM,ScholerHR.Oct-4: Gatekeeper in the beginnings of mammalian development[J].Stem Cells,2001,19(4):271-278.
doi: 10.1634/stemcells.19-4-271 |
| 17 |
CharronF,ParadisP,BronchainO,et al.Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression[J].Mol Cell Biol,1999,19(6):4355-4365.
doi: 10.1128/MCB.19.6.4355 |
| 18 | 卜萌萌. 中胚层细胞分化新功能基因的鉴定及功能研究[D]. 北京: 北京协和医学院, 2020. |
| 19 |
SchlutermanMK,KrysiakAE,KathiriyaIS,et al.Screening and biochemical analysis of GATA4 sequence variations identified in patients with congenital heart disease[J].Am J Med Genet A,2007,143A(8):817-823.
doi: 10.1002/ajmg.a.31652 |
| 20 |
zur NiedenNI,KempkaG,AhrHJ.Molecular multiple endpoint embryonic stem cell test: A possible approach to test for the teratogenic potential of compounds[J].Toxicol Appl Pharmacol,2004,194(3):257-269.
doi: 10.1016/j.taap.2003.09.019 |
| 21 |
GordeevaO,GordeevA.Comparative assessment of toxic responses in 3D embryoid body differentiation model and mouse early embryos treated with 5-hydroxytryptophan[J].Arch Toxicol,2021,95(1):253-269.
doi: 10.1007/s00204-020-02909-w |
| 22 |
LiangS,ZhouH,YinN,et al.Embryoid body-based RNA-seq analyses reveal a potential TBBPA multifaceted developmental toxicity[J].J Hazard Mater,2019,376,223-232.
doi: 10.1016/j.jhazmat.2019.05.030 |
| 23 |
KimI Q,MarikawaY.Embryoid body test with morphological and molecular endpoints implicates potential developmental toxicity of trans-resveratrol[J].Toxicol Appl Pharmacol,2018,355,211-225.
doi: 10.1016/j.taap.2018.07.006 |
| 24 |
LeeJH,ParkSY,AhnC,et al.Pre-validation study of alternative developmental toxicity test using mouse embryonic stem cell-derived embryoid bodies[J].Food Chem Toxicol,2019,123,50-56.
doi: 10.1016/j.fct.2018.10.044 |
| 25 | LeeJH,ParkSY,AhnC,et al.Second-phase validation study of an alternative developmental toxicity test using mouse embryonic stem cell-derived embryoid bodies[J].J Physiol Pharmacol,2020,71(2):223-233. |
| 26 |
夏荃,鲍倩,蒋德菊,等.基于小鼠胚胎干细胞实验模型评价川芎水煎液的胚胎毒性[J].中成药,2018,40(9):1910-1915.
doi: 10.3969/j.issn.1001-1528.2018.09.003 |
| 27 |
van OostromCT,SlobW,van der VenLT.Defining embryonic developmental effects of chemical mixtures using the embryonic stem cell test[J].Food Chem Toxicol,2020,140,111284.
doi: 10.1016/j.fct.2020.111284 |
| 28 |
ArisA,LeblancS.Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada[J].Reprod Toxicol,2011,31(4):528-533.
doi: 10.1016/j.reprotox.2011.02.004 |
| 29 |
WalshMC,BuzoianuSG,ReaMC,et al.Effects of feeding Bt MON810 maize to pigs for 110 days on peripheral immune response and digestive fate of the cry1Ab gene and truncated Bt toxin[J].PLoS One,2012,7(5):e36141.
doi: 10.1371/journal.pone.0036141 |
| 30 |
ShimadaN,KimYS,MiyamotoK,et al.Effects of Bacillus thuringiensis Cry1Ab toxin on mammalian cells[J].J Vet Med Sci,2003,65(2):187-191.
doi: 10.1292/jvms.65.187 |
| 31 |
BondzioA,StumpffF,SchönJ,et al.Impact of Bacillus thurin-giensis toxin Cry1Ab on rumen epithelial cells (REC): A new in vitro model for safety assessment of recombinant food compounds[J].Food Chem Toxicol,2008,46(6):1976-1984.
doi: 10.1016/j.fct.2008.01.038 |
| 32 |
BondzioA,LodemannU,WeiseC,et al.Cry1Ab treatment has no effects on viability of cultured porcine intestinal cells, but triggers Hsp70 expression[J].PLoS One,2013,8(7):e67079.
doi: 10.1371/journal.pone.0067079 |
| 33 | 郭梦凡,韩超,李岩,等.转Cry1Ab和epsps基因抗虫耐除草剂玉米对三代繁殖大鼠子代的神经行为与认知能力的影响[J].卫生研究,2018,47(3):419-424. |
| 34 |
LiZ,GaoY,ZhangM,et al.Effects of a diet containing genetically modified rice expressing the Cry1Ab/1Ac protein (Bacillus thuringiensis toxin) on broiler chickens[J].Arch Anim Nutr,2015,69(6):487-498.
doi: 10.1080/1745039X.2015.1087749 |
| 35 |
BuzoianuSG,WalshMC,ReaMC,et al.Transgenerational effects of feeding genetically modified maize to nulliparous sows and offspring on offspring growth and health[J].J Anim Sci,2013,91(1):318-330.
doi: 10.2527/jas.2012-5360 |
| 36 | RidingsJE.The thalidomide disaster, lessons from the past[J].Methods Mol Biol,2013,947,575-586. |
| 37 |
NiraulaPM,FondongVN.Development and adoption of geneti-cally engineered plants for virus resistance: Advances, opportunities and challenges[J].Plants,2021,10(11):2339.
doi: 10.3390/plants10112339 |
| [1] | Yuan PAN,Hang GU,Han XIAO,Lijun ZHAO,Yiman TANG,Wenshu GE. Ubiquitin-specific protease 42 regulates osteogenic differentiation of human adipose-derived stem cells [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 9-16. |
| [2] | ZHANG Sheng-nan,AN Na,OUYANG Xiang-ying,LIU Ying-jun,WANG Xue-kui. Role of growth arrest-specific protein 6 in migration and osteogenic differentiation of human periodontal ligament cells [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 9-15. |
| [3] | SUI Hua-xin, LV Pei-jun, WANG Yong, FENG Yu-chi. Effects of low level laser irradiation on the osteogenic capacity of sodium alginate/gelatin/human adipose-derived stem cells 3D bio-printing construct [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 868-875. |
| [4] | SUI Hua-xin, LV Pei-jun, WANG Yu-guang, WANG Yong, SUN Yu-chun. Effect of lowlevel laser irradiation on proliferation and osteogenic differentiation of human adipose-derived stromal cells [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 337-343. |
| [5] | LING Long, ZHAO Yu-ming, GE Li-hong. Impact of different degree pulpitis on cell proliferation and osteoblastic differentiation of dental pulp stem cell in Beagle immature premolars [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 878-883. |
| [6] | GE Wen-shu, TANG Yi-man, ZHANG Xiao, LIU Yun-song, ZHOU Yong-sheng. Establishing a luciferase reporter system to evaluate osteogenic differentiation potential of human adipose-derived stem cells [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 170-174. |
| [7] | ZHANG Xiao, LIU Yun-song, LV Long-wei, CHEN Tong, WU Gang, ZHOU Yong-sheng. Promoted role of bone morphogenetic protein 2/7 heterodimer in the osteogenic differentiation of human adipose-derived stem cells [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 37-44. |
| [8] | YU Guang-yan, CAO Tong, ZOU Xiao-hui, ZHANG Xue-hui,FU Xin, PENG Shuang-qing, DENG Xu-liang, LI Sheng-lin, LIU He, XIAO Ran, OUYANG Hong-wei, PENG Hui, CHEN Xiao, ZHAO Zeng-ming, WANG Xiao-ying……. Development of human embryonic stem cell platforms for human health-safety #br# evaluation [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 1-4. |
| [9] | WANG Xiao-fei, LV Pei-jun, SONG Yang, WANG Yong, SUN Yu-chun. Short-term effect of CaCl2 on human adipose-derived mesenchymal stem cells proliferation and osteogenic differentiation [J]. Journal of Peking University(Health Sciences), 2015, 47(6): 971-976. |
| [10] | JIA Shuang-shuang, LI Wei-yang, LIU Xin, LI Li-ying. Transforming growth factor-β1 induces differentiation of bone marrowderived mesenchymal stem cells into myofibroblasts via production of reactive oxygen species [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 737-742. |
| [11] | SONG Yi-Meng, MA Lu-Lin, LI Xiao-Xia, ZHU Yi. Mechanisms of prostaglandin E2-induced bone marrow-derived progenitor cell differentiation [J]. Journal of Peking University(Health Sciences), 2015, 47(4): 577-581. |
| [12] | XU Xiang-liang, WANG En-bo, CUI Nian-hui. Influence of acellular dermal matrix on differentiation of stem cells from young permanent tooth apical papilla [J]. Journal of Peking University(Health Sciences), 2014, 46(1): 12-18. |
| [13] | DING Qian, ZHANG Feng-qiu, MA Yu-shi. Effect of icariin on osteoblastic differentiation gene expression of human periodontal ligament cells [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 975-978. |
|
||