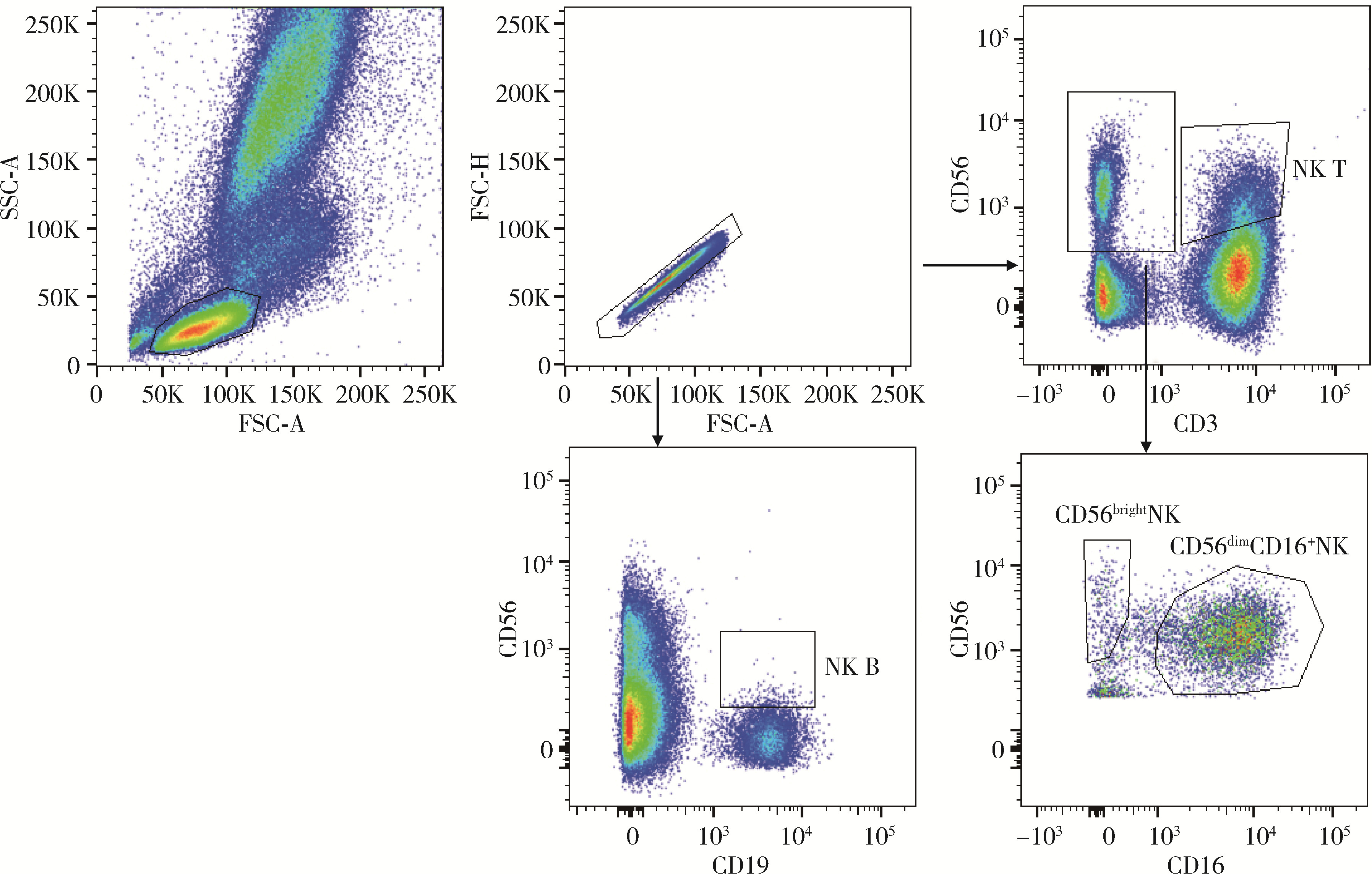
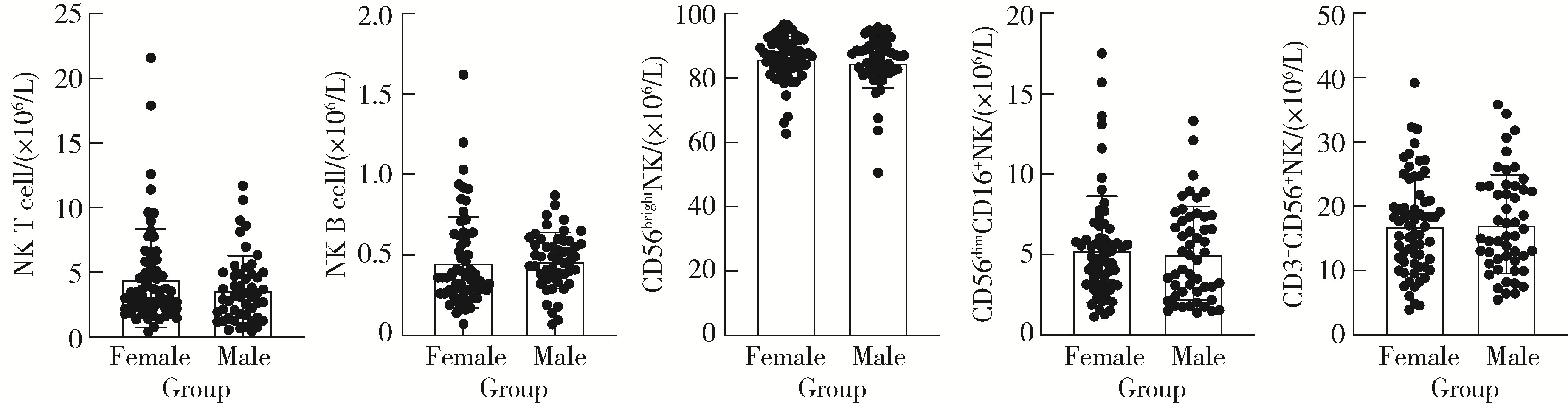
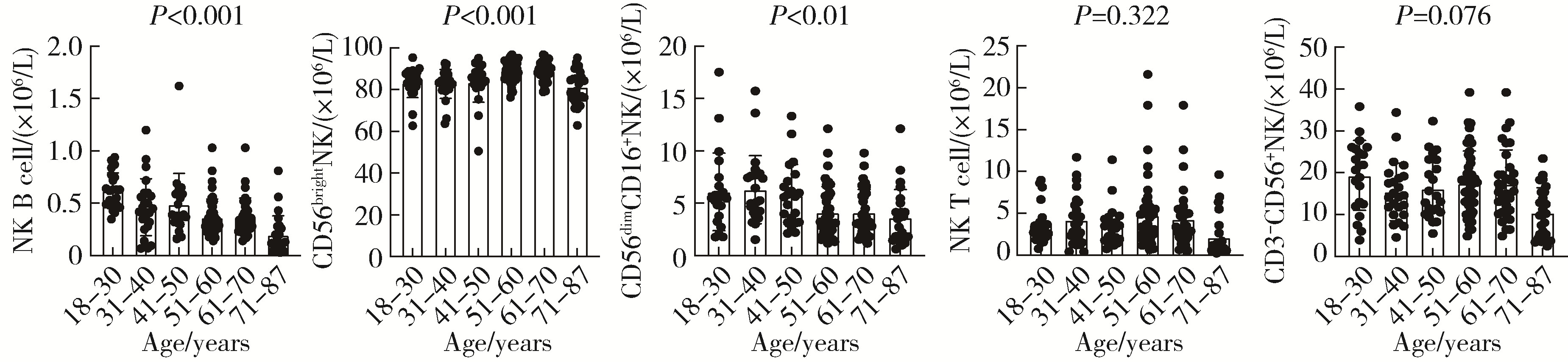
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (5): 839-844. doi: 10.19723/j.issn.1671-167X.2024.05.014
Previous Articles Next Articles
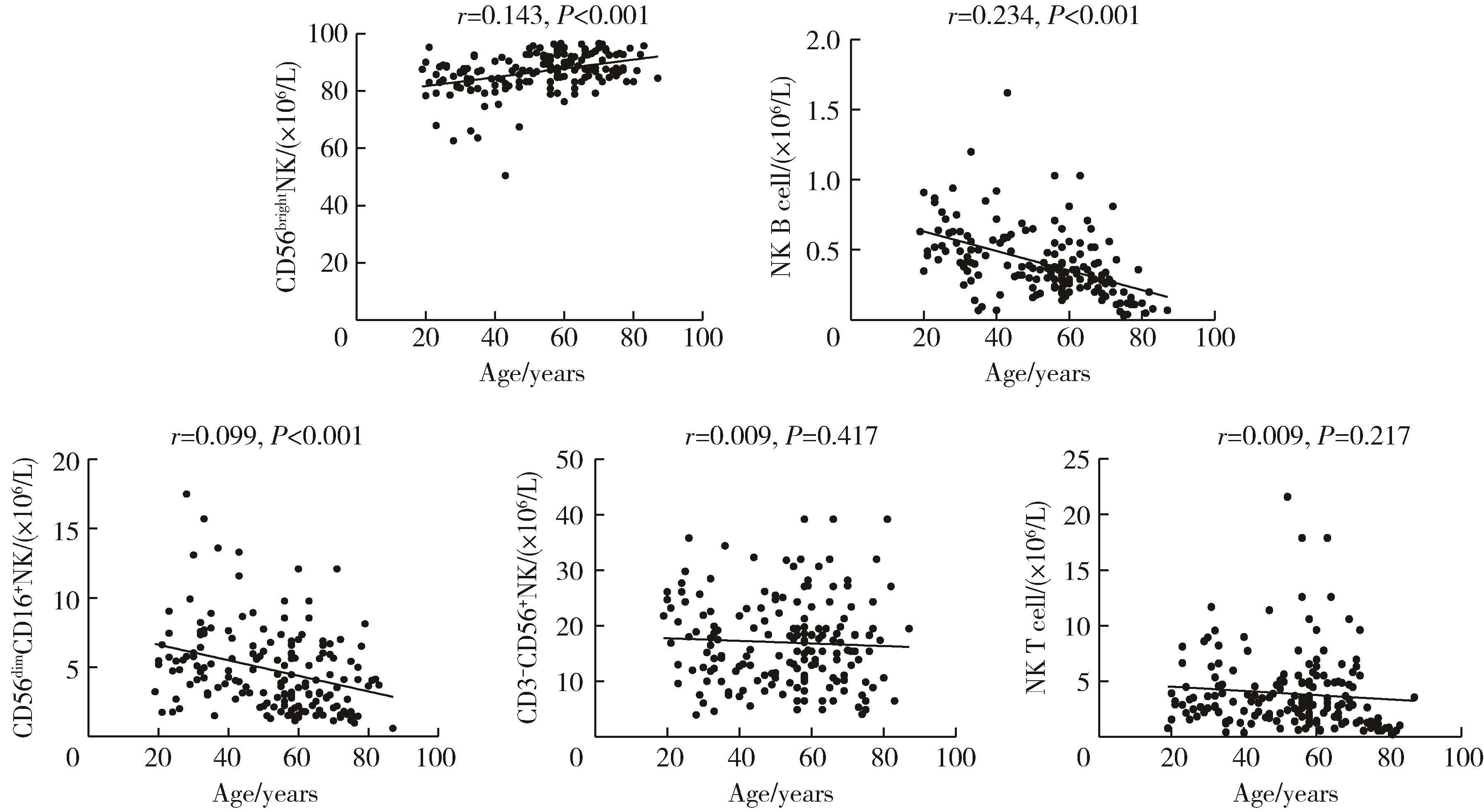
Flow cytometry analysis of normal range of natural killer cells and their subsets in peripheral blood of healthy Chinese adults
Jiayi TIAN, Yixue GUO, Xia ZHANG, Xiaolin SUN, Jing HE*( )
)
- Department of Rheumatology and Immunology, Peking University People's Hospital, Beijing 100044, China
CLC Number:
- R593.2
| 1 | Yu J , Freud AG , Caligiuri MA . Location and cellular stages of natural killer cell development[J]. Trends Immunol, 2013, 34 (12): 573- 582. |
| 2 | Carrega P , Ferlazzo G . Natural killer cell distribution and trafficking in human tissues[J]. Front Immunol, 2012, 3, 347. |
| 3 | Zhu L , Karakizlis H , Weimer R , et al. Circulating NKG2A-NKG2D+ CD56dimCD16+ natural killer (NK) cells as mediators of functional immunosurveillance in kidney transplant recipients[J]. Ann Transplant, 2020, 25, e925162. |
| 4 | Marras F , Casabianca A , Bozzano F , et al. Control of the HIV-1 DNA reservoir is associated in vivo and in vitro with NKp46/NKp30 (CD335 CD337) inducibility and interferon gamma production by transcriptionally unique NK cells[J]. J Virol, 2017, 91 (23): e00647. |
| 5 | Cichocki F , Grzywacz B , Miller JS . Human NK cell development: One road or many?[J]. Front Immunol, 2019, 10, 2078. |
| 6 | Freud AG , Mundy-Bosse BL , Yu J , et al. The broad spectrum of human natural killer cell diversity[J]. Immunity, 2017, 47 (5): 820- 833. |
| 7 | Melzer S , Zachariae S , Bocsi J , et al. Reference intervals for leukocyte subsets in adults: Results from a population-based study using 10-color flow cytometry[J]. Cytometry B Clin Cytom, 2015, 88 (4): 270- 281. |
| 8 | Shu SA , Wang J , Tao MH , et al. Gene therapy for autoimmune disease[J]. Clin Rev Allergy Immunol, 2015, 49 (2): 163- 176. |
| 9 | Choi J , Lee SJ , Lee YA , et al. Reference values for peripheral blood lymphocyte subsets in a healthy korean population[J]. Immune Netw, 2014, 14 (6): 289- 295. |
| 10 | Yawata N , Selva KJ , Liu YC , et al. Dynamic change in natural killer cell type in the human ocular mucosa in situ as means of immune evasion by adenovirus infection[J]. Mucosal Immunol, 2016, 9 (1): 159- 170. |
| 11 | Tahrali I , Kucuksezer UC , Akdeniz N , et al. CD3-CD56+ NK cells display an inflammatory profile in RR-MS patients[J]. Immunol Lett, 2019, 216, 63- 69. |
| 12 | Bendelac A , Savage PB , Teyton L . The biology of NK T cells[J]. Annu Rev Immunol, 2007, 25, 297- 336. |
| 13 | McCarthy C , Shepherd D , Fleire S , et al. The length of lipids bound to human CD1d molecules modulates the affinity of NK T cell TCR and the threshold of NK T cell activation[J]. J Exp Med, 2007, 204 (5): 1131- 1144. |
| 14 | Simoni Y , Diana J , Ghazarian L , et al. Therapeutic manipulation of natural killer (NK) T cells in autoimmunity: Are we close to reality?[J]. Clin Exp Immunol, 2013, 171 (1): 8- 19. |
| 15 | Li M , Xiong Y , Li M , et al. Depletion but activation of CD56(dim)CD16(+)NK cells in acute infection with severe fever with thrombocytopenia syndrome virus[J]. Virol Sin, 2020, 35 (5): 588- 598. |
| 16 | Gayoso I , Sanchez-Correa B , Campos C , et al. Immunosene-scence of human natural killer cells[J]. J Innate Immun, 2011, 3 (4): 337- 343. |
| 17 | Solana R , Tarazona R , Gayoso I , et al. Innate immunosene-scence: Effect of aging on cells and receptors of the innate immune system in humans[J]. Semin Immunol, 2012, 24 (5): 331- 341. |
| 18 | Solana R , Pawelec G , Tarazona R . Aging and innate immunity[J]. Immunity, 2006, 24 (5): 491- 494. |
| 19 | Cunha CF , Ferraz-Nogueira R , Costa VFA , et al. Contribution of Leishmania braziliensis antigen-specific CD4+ T, CD8+ T, NK and CD3+CD56+NK T cells in the immunopathogenesis of cutaneous leishmaniasis patients: Cytotoxic, activation and exhaustion profiles[J]. PLoS One, 2020, 15 (3): e0229400. |
| 20 | Myers JA , Miller JS . Exploring the NK cell platform for cancer immunotherapy[J]. Nat Rev Clin Oncol, 2021, 18 (2): 85- 100. |
| 21 | Terrén I , Orrantia A , Vitallé J , et al. NK Cell metabolism and tumor microenvironment[J]. Frontiers in immunology, 2019, 10, 2278. |
| 22 | Zhang C , Liu Y . Targeting NK Cell Checkpoint receptors or molecules for cancer immunotherapy[J]. Front Immunol, 2020, 11, 1295. |
| [1] | Xiang-ge ZHAO,Jia-qing LIU,Hui-na HUANG,Zhi-min LU,Zi-ran BAI,Xia LI,Jing-jing QI. Interferon-α mediating the functional damage of CD56dimCD57+natural killer cells in peripheral blood of systemic lupus erythematosuss [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 975-981. |
| [2] | MA Xiang-bo,ZHANG Xue-wu,JIA Ru-lin,GAO Ying,LIU Hong-jiang,LIU Yu-fang,LI Ying-ni. Application of lymphocytes test in peripheral blood of patients with systemic sclerosis during the treatment [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 721-727. |
| [3] | Yun-bo XIE,Ji-yuan ZHANG,Mei-ling DU,Fan-ping MENG,Jun-liang FU,Li-min LIU,Song-shan WANG,Rui QU,Fang LIAN,Fei QIAO,Yang-liu CHEN,Ying-ying GAO,Ruo-nan XU,Ming SHI,Fu-sheng WANG. Efficacy and peripheral immunity analysis of allogeneic natural killer cells therapy in patients with hepatocellular carcinoma [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 591-595. |
|
||