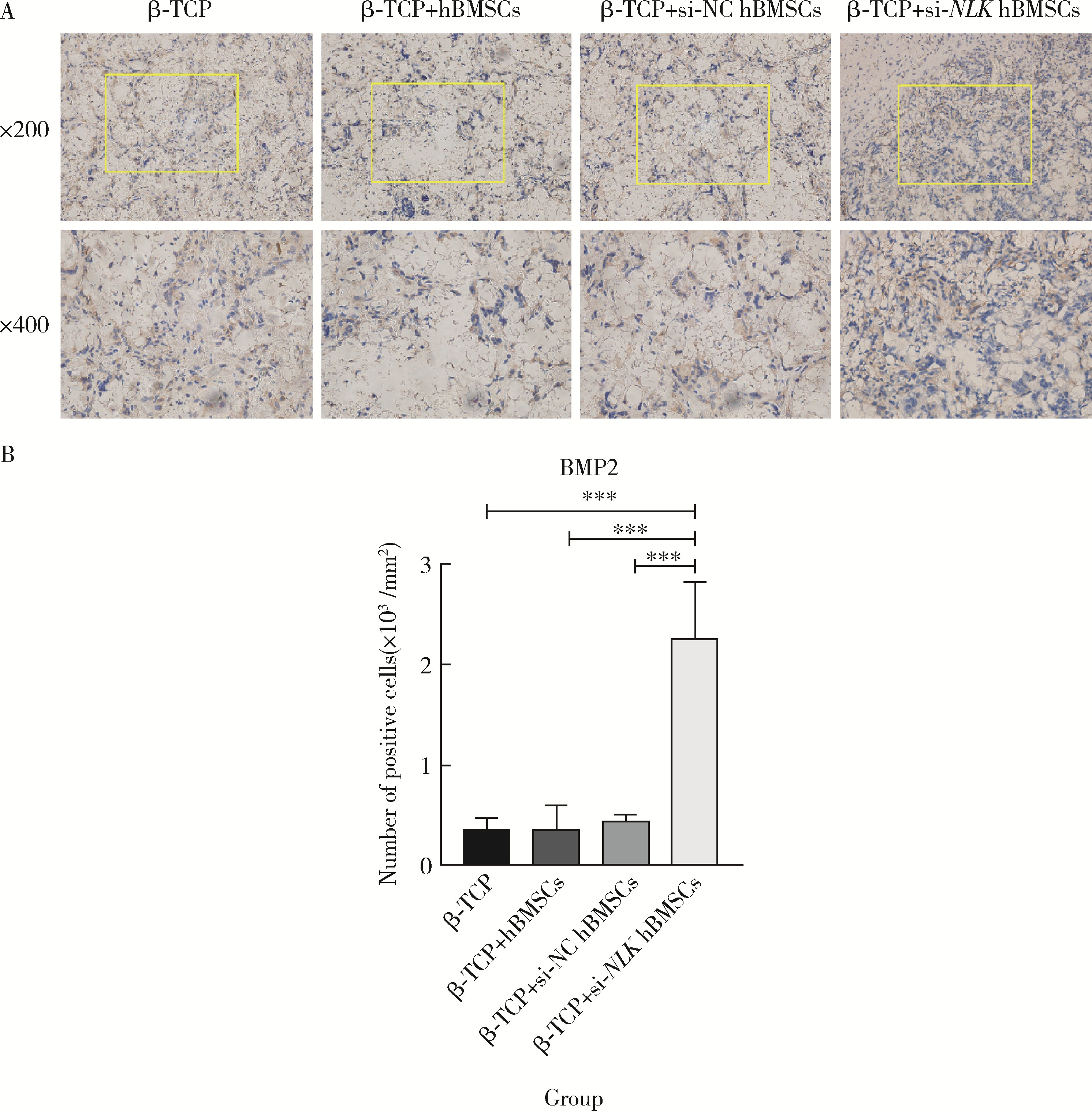
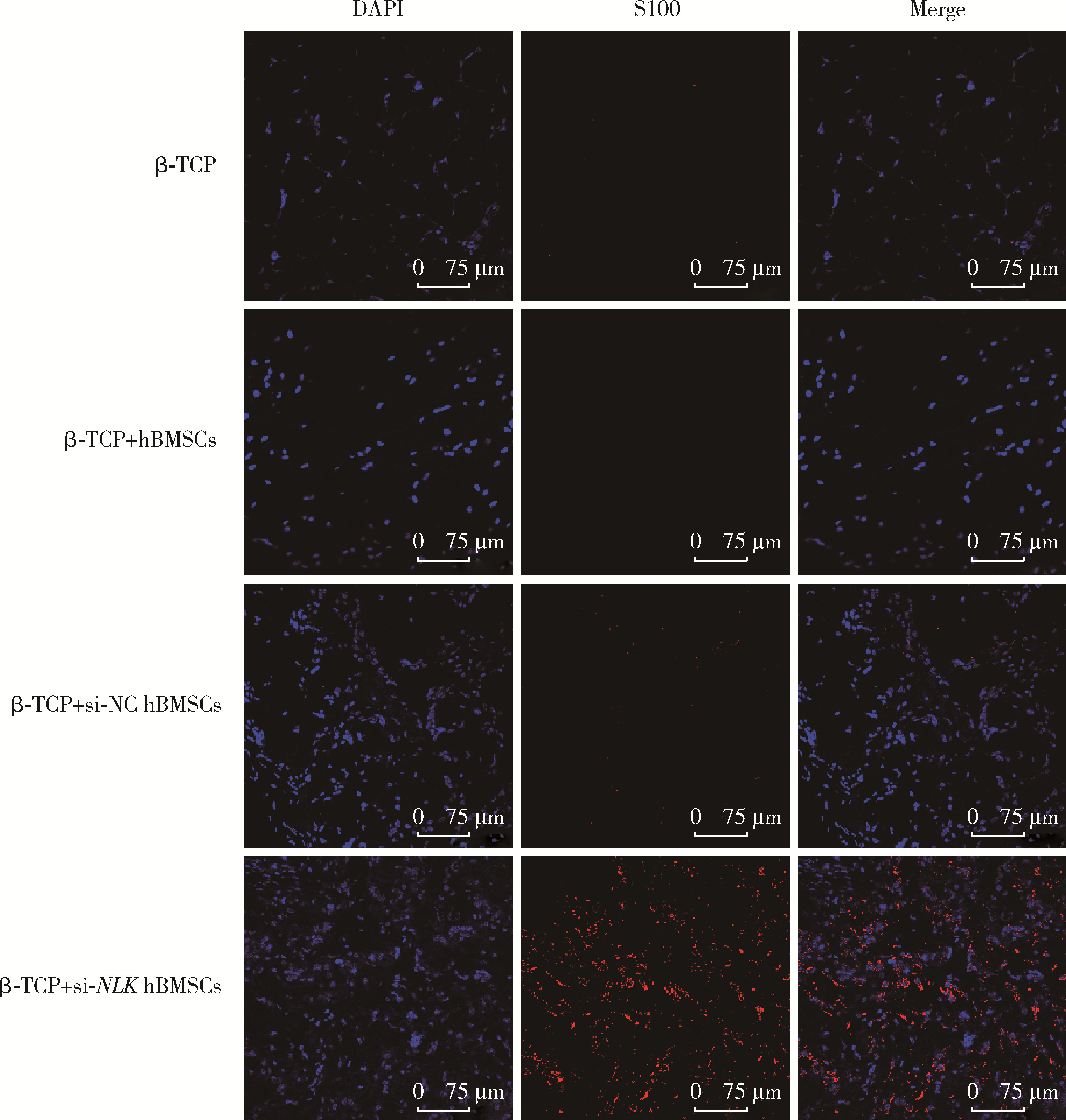
Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (2): 227-236. doi: 10.19723/j.issn.1671-167X.2025.02.002
Previous Articles Next Articles
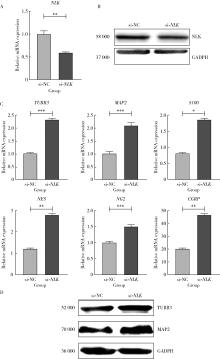
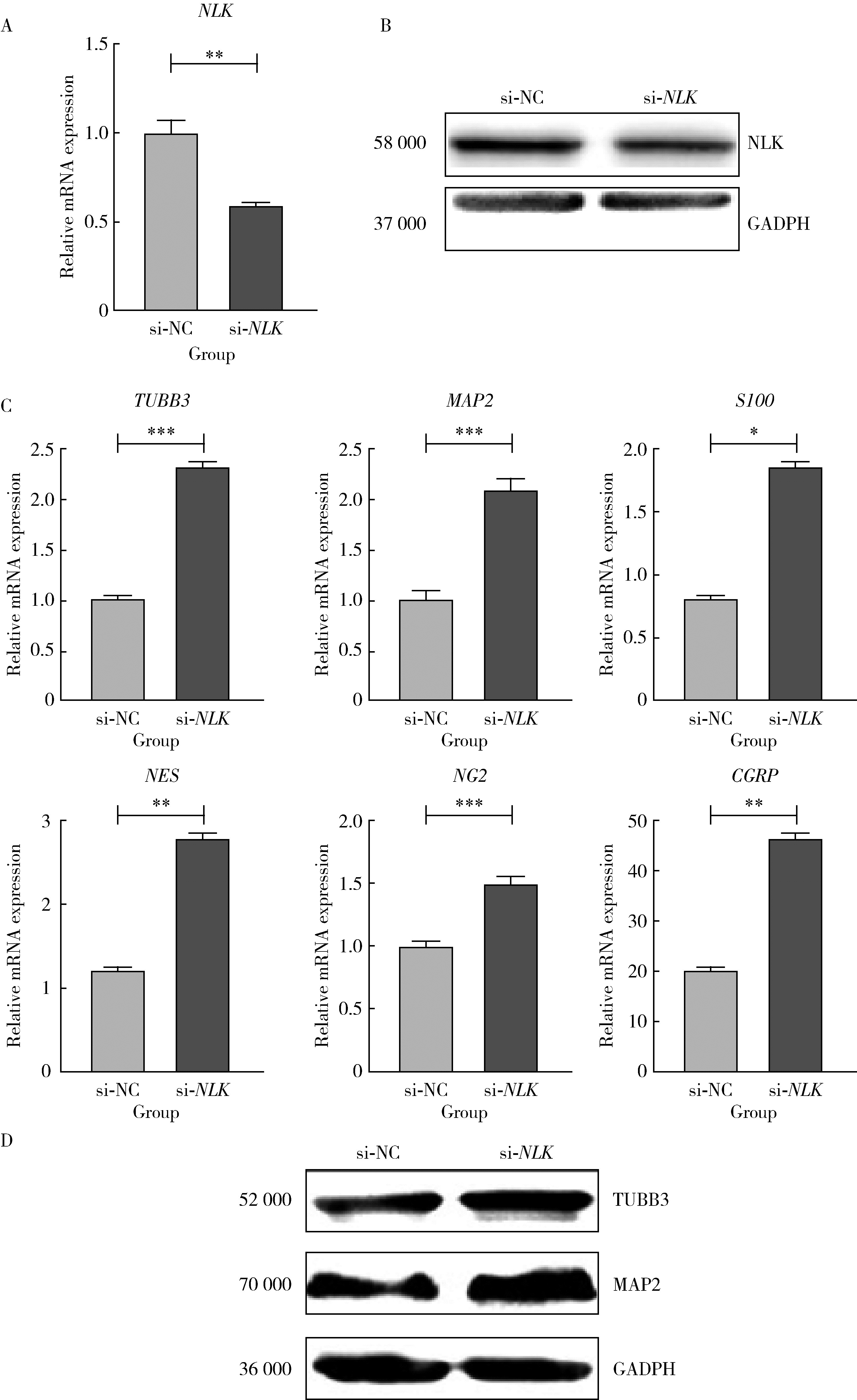
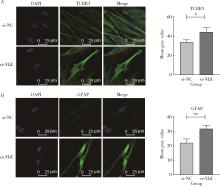
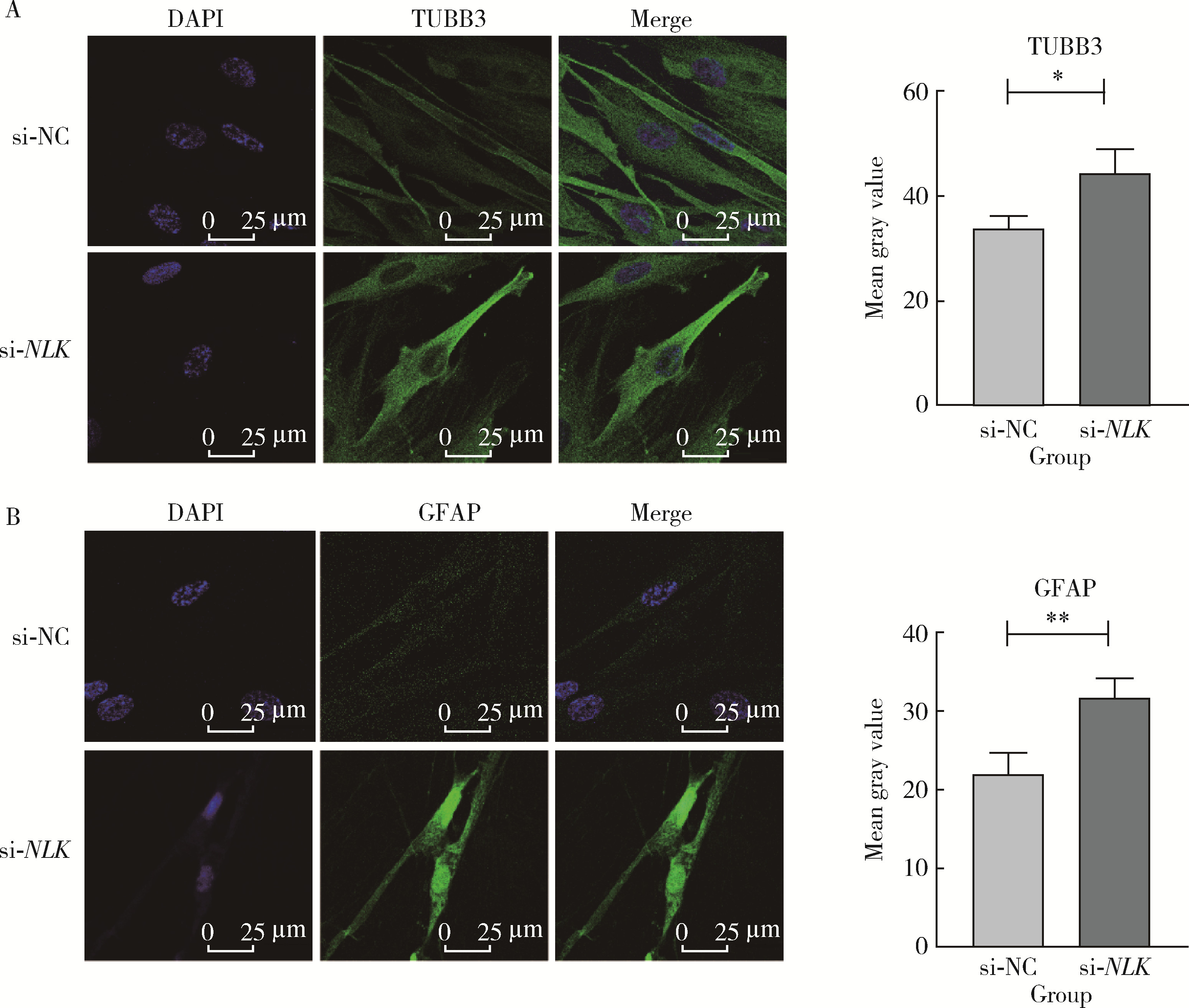
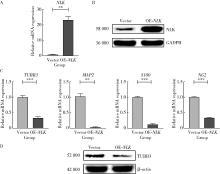
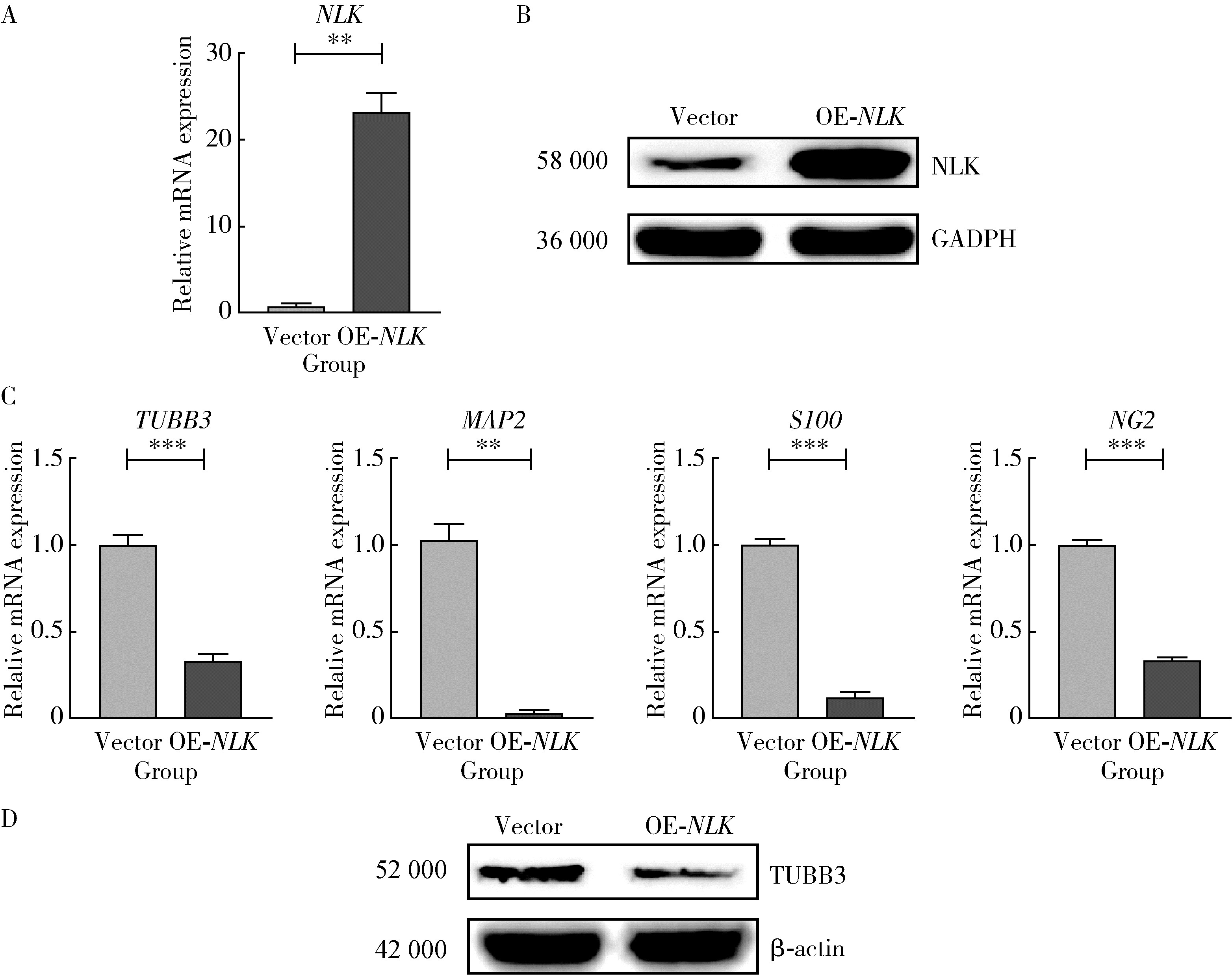
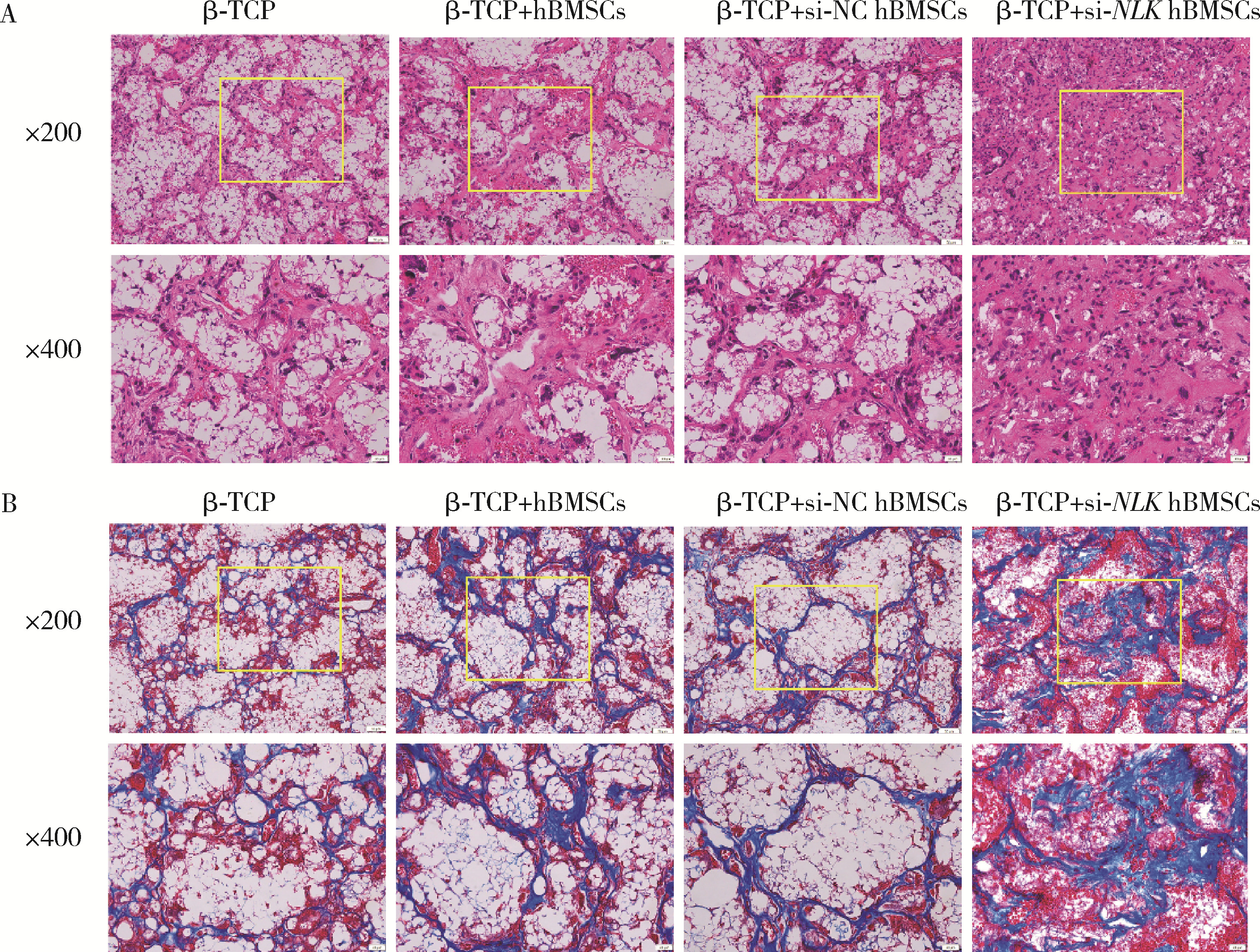
Gene silencing of Nemo-like kinase promotes neuralized tissue engineered bone regeneration
Mengdi LI1, Lei LEI2, Zhongning LIU1,*( ), Jian LI1, Ting JIANG1,*(
), Jian LI1, Ting JIANG1,*( )
)
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices, Beijing 100081, China
2. Department of Stomatology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
CLC Number:
- R783.3
| 1 |
Grässel SG . The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology[J]. Arthritis Res Ther, 2014, 16 (6): 485.
doi: 10.1186/s13075-014-0485-1 |
| 2 |
Maryanovich M , Takeishi S , Frenette PS . Neural regulation of bone and bone marrow[J]. Cold Spring Harb Perspect Med, 2018, 8 (9): a031344.
doi: 10.1101/cshperspect.a031344 |
| 3 |
Chen H , Hu B , Lv X , et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis[J]. Nat Commun, 2019, 10 (1): 181.
doi: 10.1038/s41467-018-08097-7 |
| 4 |
Li Z , Meyers CA , Chang L , et al. Fracture repair requires TrkA signaling by skeletal sensory nerves[J]. J Clin Invest, 2019, 129 (12): 5137- 5150.
doi: 10.1172/JCI128428 |
| 5 | Wu Y , Jing D , Ouyang H , et al. Pre-implanted sensory nerve could enhance the neurotization in tissue-engineered bone graft[J]. Tissue Eng Part A, 2015, 21 (15/16): 2241- 2249. |
| 6 |
Zhang Y , Xu J , Ye CR , et al. Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats[J]. Nat Med, 2016, 22 (10): 1160- 1169.
doi: 10.1038/nm.4162 |
| 7 | Liu Q , Lei L , Yu T , et al. Effect of brain-derived neurotrophic factor on the neurogenesis and osteogenesis in bone engineering[J]. Tissue Eng Part A, 2018, 24 (15/16): 1283- 1292. |
| 8 | 覃俊君, 尹东, 裴国献, 等. 植入单纯血管束、感觉神经束的组织工程骨修复大段骨缺损[J]. 中国组织工程研究, 2017, 21 (8): 1161- 1166. |
| 9 | 陈思圆, 王簕, 江汕, 等. 植入含感觉神经束和血管束组织工程骨修复股骨大段骨缺损: 降钙素基因相关肽Ⅰ型受体的中长期表达[J]. 中国组织工程研究, 2017, 21 (6): 848- 853. |
| 10 | Yu B , Zhou S , Wang Y , et al. miR-221 and miR-222 promote Schwann cell proliferation and migration by targeting LASS2 after sciatic nerve injury[J]. J Cell Sci, 2012, 125 (Pt 11): 2675- 2683. |
| 11 |
Song J , Li X , Li Y , et al. Biodegradable and biocompatible cationic polymer delivering microRNA-221/222 promotes nerve regeneration after sciatic nerve crush[J]. Int J Nanomedicine, 2017, 12, 4195- 4208.
doi: 10.2147/IJN.S132190 |
| 12 |
Lei L , Liu Z , Yuan P , et al. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of micro-RNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects[J]. J Mater Chem B, 2019, 7 (16): 2722- 2735.
doi: 10.1039/C9TB00025A |
| 13 |
Ishitani T , Ishitani S . Nemo-like kinase, a multifaceted cell signaling regulator[J]. Cell Signal, 2013, 25 (1): 190- 197.
doi: 10.1016/j.cellsig.2012.09.017 |
| 14 |
Nifuji A , Ideno H , Ohyama Y , et al. Nemo-like kinase (NLK) expression in osteoblastic cells and suppression of osteoblastic differentiation[J]. Exp Cell Res, 2010, 316 (7): 1127- 1136.
doi: 10.1016/j.yexcr.2010.01.023 |
| 15 |
Zanotti S , Canalis E . Nemo-like kinase inhibits osteoblastogenesis by suppressing bone morphogenetic protein and WNT canonical signaling[J]. J Cell Biochem, 2012, 113 (2): 449- 456.
doi: 10.1002/jcb.23365 |
| 16 |
Fakhr E , Zare F , Teimoori-Toolabi L . Precise and efficient siRNA design: A key point in competent gene silencing[J]. Cancer Gene Ther, 2016, 23 (4): 73- 82.
doi: 10.1038/cgt.2016.4 |
| 17 |
Sun X , Peng X , Cao Y , et al. ADNP promotes neural differentiation by modulating Wnt/β-catenin signaling[J]. Nat Commun, 2020, 11 (1): 2984.
doi: 10.1038/s41467-020-16799-0 |
| 18 |
Tawk M , Makoukji J , Belle M , et al. Wnt/beta-catenin signaling is an essential and direct driver of myelin gene expression and myelinogenesis[J]. J Neurosci, 2011, 31 (10): 3729- 3742.
doi: 10.1523/JNEUROSCI.4270-10.2011 |
| 19 |
Kodama D , Hirai T , Kondo H , et al. Bidirectional communication between sensory neurons and osteoblasts in an in vitro coculture system[J]. FEBS Lett, 2017, 591 (3): 527- 539.
doi: 10.1002/1873-3468.12561 |
| 20 |
Burbach JP . Neuropeptides from concept to online database[J]. Eur J Pharmacol, 2010, 626 (1): 27- 48.
doi: 10.1016/j.ejphar.2009.10.015 |
| 21 |
龙域丰, 朱古力, 易伟宏, 等. 神经肽类物质在骨代谢与骨再生中的调控作用[J]. 中华实验外科杂志, 2020, 37 (11): 2131- 2136.
doi: 10.3760/cma.j.cn421213-20191020-00747 |
| 22 |
He H , Chai J , Zhang S , et al. CGRP may regulate bone metabolism through stimulating osteoblast differentiation and inhibiting osteoclast formation[J]. Mol Med Rep, 2016, 13 (5): 3977- 3984.
doi: 10.3892/mmr.2016.5023 |
| 23 |
Chen J , Liu W , Zhao J , et al. Gelatin microspheres containing calcitonin gene-related peptide or substance P repair bone defects in osteoporotic rabbits[J]. Biotechnol Lett, 2017, 39 (3): 465- 472.
doi: 10.1007/s10529-016-2263-4 |
| 24 | Niedermair T , Kuhn V , Doranehgard F , et al. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification[J]. Matrix Biol, 2014, 9 (38): 22- 35. |
| 25 |
Song L , Jin D , Wu JQ , et al. Neuropeptide Y stimulates osteoblastic differentiation and VEGF expression of bone marrow mesenchymal stem cells related to canonical Wnt signaling activating in vitro[J]. Neuropeptides, 2016, 56, 105- 113.
doi: 10.1016/j.npep.2015.12.008 |
| 26 | Zhang S , Li J , Jiang H , et al. Dorsal root ganglion maintains stemness of bone marrow mesenchymal stem cells by enhancing autophagy through the AMPK/mTOR pathway in a coculture system[J]. Stem Cells Int, 2018, 2018, 8478953. |
| [1] | Mei WANG, Bo-wen LI, Si-wen WANG, Yu-hua LIU. Preparation and osteogenic effect study of small intestinal submucosa sponge [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 952-958. |
| [2] | Rong LI,Ke-long CHEN,Yong WANG,Yun-song LIU,Yong-sheng ZHOU,Yu-chun SUN. Establishment of a 3D printing system for bone tissue engineering scaffold fabrication and the evaluation of its controllability over macro and micro structure precision [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 115-119. |
|
||