Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (5): 952-958. doi: 10.19723/j.issn.1671-167X.2020.05.027
Previous Articles Next Articles
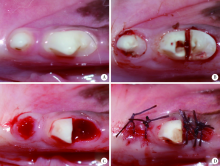
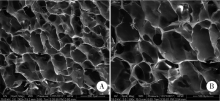
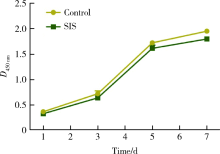
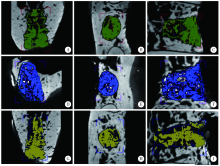
Preparation and osteogenic effect study of small intestinal submucosa sponge
Mei WANG1*,Bo-wen LI1*,Si-wen WANG2,Yu-hua LIU1,∆( )
)
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. Department of Prosthodontics, Tongji University School and Hospital of Stomatology, Shanghai 200072, China
CLC Number:
- R783.3
| [1] |
Avilaortiz G, Elangovan S, Kramer KWO, et al. Effect of alveolar ridge preservation after tooth extraction: A systematic review and meta-analysis[J]. J Dent Res, 2014,93(10):950-958.
doi: 10.1177/0022034514541127 |
| [2] |
López M, Fanny, Gómez M, et al. Implants failures related to endodontic treatment. An observational retrospective study[J]. Clin Oral Implant Res, 2015,26(9):992-995.
doi: 10.1111/clr.2015.26.issue-9 |
| [3] |
Horváth A, Mardas N, Mezzomo LA, et al. Alveolar ridge preservation. A systematic review[J]. Clin Oral Investig, 2013,17(2):341-363.
doi: 10.1007/s00784-012-0758-5 pmid: 22814758 |
| [4] |
Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds[J]. Trends Biotechnol, 2012,30(10):546-554.
doi: 10.1016/j.tibtech.2012.07.005 |
| [5] | 蒋欣泉. 骨缺损修复生物材料与骨再生[J]. 中华口腔医学杂志, 2017,52(10):600-604. |
| [6] |
Andrée B, Bär A, Haverich A, et al. Small intestinal submucosa segments as matrix for tissue engineering: review[J]. Tissue Eng Part B, 2013,19(4):279-291.
doi: 10.1089/ten.teb.2012.0583 |
| [7] |
Nezhad ZM, Poncelet A, Kerchove LD, et al. Small intestinal submucosa extracellular matrix (CorMatrix®) in cardiovascular surgery: A systematic review [J]. Interact Cardiovasc Thorac Surg, 2016,22(6):839-850.
doi: 10.1093/icvts/ivw020 pmid: 26912574 |
| [8] |
Li M, Zhang C, Mao Y, et al. A cell-engineered small intestinal submucosa-based bone mimetic construct for bone regeneration[J]. Tissue Eng Part A, 2018,24(13):1099-1111.
doi: 10.1089/ten.tea.2017.0407 |
| [9] | 房艳, 倪伟民, 单伟, 等. 海绵状的小肠粘膜下层促进成骨样细胞增殖分化[J]. 中国生物工程杂志, 2013,33(6):18-23. |
| [10] |
Kim KS, Lee J Y, Kang YM, et al. Small intestine submucosa sponge for in vivo support of tissue-engineered bone formation in the presence of rat bone marrow stem cells[J]. Biomaterials, 2010,31(6):1104-1113.
doi: 10.1016/j.biomaterials.2009.10.020 |
| [11] |
Lin X, Chen J, Qiu P, et al. Biphasic hierarchical extracellular matrix scaffold for osteochondral defect regeneration[J]. Osteoarthritis Cartilage, 2018,26(3):433-444.
doi: 10.1016/j.joca.2017.12.001 pmid: 29233641 |
| [12] | Cunniffe GM, Díazpayno PJ, Ramey JS, et al. Growth plate extracellular matrix-derived scaffolds for large bone defect healing[J]. Eur Cells Mater, 2017,33(1):130-142. |
| [13] |
Wang W, Zhang X, Chao NN, et al. Preparation and charac-terization of proangiogenic gel derived from small intestinal submucosa[J]. Acta Biomaterialia, 2016,29(1):135-148.
doi: 10.1016/j.actbio.2015.10.013 |
| [14] |
Lin X, Robinson M, Petrie T, et al. Small intestinal submucosa-derived extracellular matrix bioscaffold significantly enhances angiogenic factor secretion from human mesenchymal stromal cells[J]. Stem Cell Res Ther, 2015,6(1):164-176.
doi: 10.1186/s13287-015-0165-3 |
| [15] |
Kim MS, Hong KD, Shin HW, et al. Preparation of porcine small intestinal submucosa sponge and their application as a wound dressing in full-thickness skin defect of rat[J]. Int J Biol Macromol, 2005,36(1/2):54-60.
doi: 10.1016/j.ijbiomac.2005.03.013 |
| [16] |
Li M, Zhang C, Cheng M, et al. Small intestinal submucosa: A potential osteoconductive and osteoinductive biomaterial for bone tissue engineering[J]. Mater Sci Eng C Biomim Supramol Syst, 2017,75(6):149-156.
doi: 10.1016/j.msec.2017.02.042 |
| [17] |
Dimitriou R, Mataliotakis GI, Calori GM, et al. The role of barrier membranes for guided bone regeneration and restoration of large bone defects: current experimental and clinical evidence[J]. BMC Med, 2012,10(1):81-105.
doi: 10.1186/1741-7015-10-81 |
| [18] |
Rouwkema J, Rivron NC, Blitterswijk CAV. Vascularization in tissue engineering[J]. Trends Biotechnol, 2008,26(8):434-441.
doi: 10.1016/j.tibtech.2008.04.009 |
| [19] |
Bolaños MAC, Buttigieg J, Triana JCB. Development and characterization of a novel porous small intestine submucosa-hydroxyapatite scaffold for bone regeneration[J]. Mater Sci Eng C Biomim Supramol Syst, 2017,72(3):519-525.
doi: 10.1016/j.msec.2016.11.113 |
| [20] | 孙慧哲, 田伟, 曾亮, 等. 猪小肠黏膜下基质海绵的制备[J]. 中国组织工程研究, 2016,20(21):3110-3116. |
| [21] |
Sarkar AD, Singhvi N, Shetty JN, et al. The local effect of alendronate with intra-alveolar collagen sponges on post extraction alveolar ridge resorption: A clinical trial[J]. J Oral Maxillofac Surg, 2015,14(2):344-356.
doi: 10.1007/s12663-014-0633-9 |
| [22] |
Gilbert TW, Stewartakers AM, Simmonsbyrd A, et al. Degradation and remodeling of small intestinal submucosa in canine achilles tendon repair[J]. J Bone Joint Surg Am, 2007,89(3):621-630.
doi: 10.2106/JBJS.E.00742 pmid: 17332112 |
| [23] | Wu W, Li B, Liu Y, et al. Effect of multilaminate small intestinal submucosa as a barrier membrane on bone formation in a rabbit mandible defect model[J]. Biomed Res Int, 2018(2):1-11. |
| [24] | Kim JJ, Schwarz F, Song HY, et al. Ridge preservation of extraction sockets with chronic pathology using Bio-Oss Collagen with or without collagen membrane: An experimental study in dogs[J]. Clin Oral Implant Res, 2017(28):727-733. |
| [25] |
Wang F, Li Q, Wang Z. A comparative study of the effect of Bio-Oss® in combination with concentrated growth factors or bone marrow-derived mesenchymal stem cells in canine sinus grafting [J]. J Oral Pathol Med, 2017,46(7):528-536.
doi: 10.1111/jop.12507 pmid: 27682609 |
| [1] | DU Wen-yu,YANG Jing-wen,JIANG Ting. Early constant observation of the effect of deferoxamine mesylate on improvement of vascularized bone regeneration in SD rat skull critical size defect model [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1171-1177. |
| [2] | WANG Jing-qi,WANG Xiao. In vivo study of strontium-doped calcium phosphate cement for biological properties [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 378-383. |
| [3] | ZHANG Sheng-nan,AN Na,OUYANG Xiang-ying,LIU Ying-jun,WANG Xue-kui. Role of growth arrest-specific protein 6 in migration and osteogenic differentiation of human periodontal ligament cells [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 9-15. |
| [4] | Ying CHEN,Zhong-ning LIU,Bo LI,Ting JIANG. Preparation of aspirin sustained-release microsphere and its in vitro releasing [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 907-912. |
| [5] | Rong LI,Ke-long CHEN,Yong WANG,Yun-song LIU,Yong-sheng ZHOU,Yu-chun SUN. Establishment of a 3D printing system for bone tissue engineering scaffold fabrication and the evaluation of its controllability over macro and micro structure precision [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 115-119. |
| [6] | SUI Hua-xin, LV Pei-jun, WANG Yong, FENG Yu-chi. Effects of low level laser irradiation on the osteogenic capacity of sodium alginate/gelatin/human adipose-derived stem cells 3D bio-printing construct [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 868-875. |
| [7] | LIU Jing-yin, CHEN Fei, GE Yan-jun, WEI Ling, PAN Shao-xia, FENG Hai-lan. Influence of implants prepared by selective laser melting on early bone healing [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 117-122. |
| [8] | CHEN Fei, PAN Shao-xia, FENG Hai-lan. Distribution and content of transforming growth factor-β1 and vascular endothelial growth factor in each layer of concentrated growth factors [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 860-865. |
| [9] | QIN Xue-yan, ZHAO Hua-xiang, ZHANG Qian, CHEN Feng, LIN Jiu-xiang. NELL-1: a novel highly efficient and specific growth factor [J]. Journal of Peking University(Health Sciences), 2016, 48(2): 380-383. |
| [10] | GE Wen-shu, TANG Yi-man, ZHANG Xiao, LIU Yun-song, ZHOU Yong-sheng. Establishing a luciferase reporter system to evaluate osteogenic differentiation potential of human adipose-derived stem cells [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 170-174. |
| [11] | SONG Yang, WANG Xiao-fei, WANG Yu-guang, SUN Yu-chun, LV Pei-jun. Osteogenesis of human adipose-derived mesenchymal stem cells-biomaterial mixture in vivo after 3D bio-printing [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 45-50. |
| [12] | 欧Meng-恩 , ZHANG Xiao, LIU Yun-Song, GE Yan-Jun, ZHOU Yong-Sheng. Ectopic osteogenesis of stromal cell-derived factor 1 combined with simvastatin loaded collagen scaffold in vivo [J]. Journal of Peking University(Health Sciences), 2015, 47(1): 47-51. |
| [13] | DING Qian, ZHANG Feng-qiu, MA Yu-shi. Effect of icariin on osteoblastic differentiation gene expression of human periodontal ligament cells [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 975-978. |
|
||