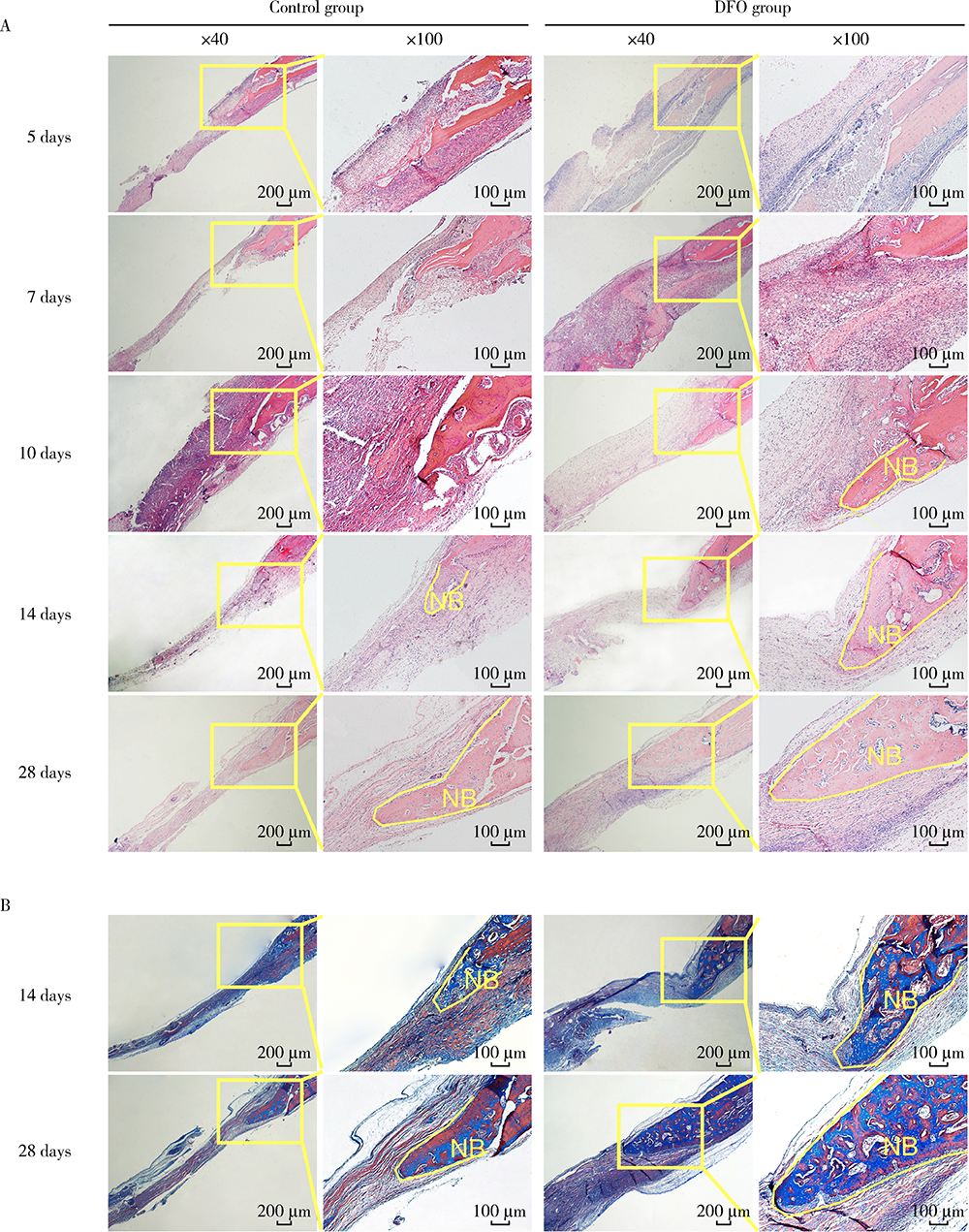
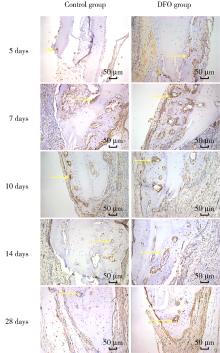
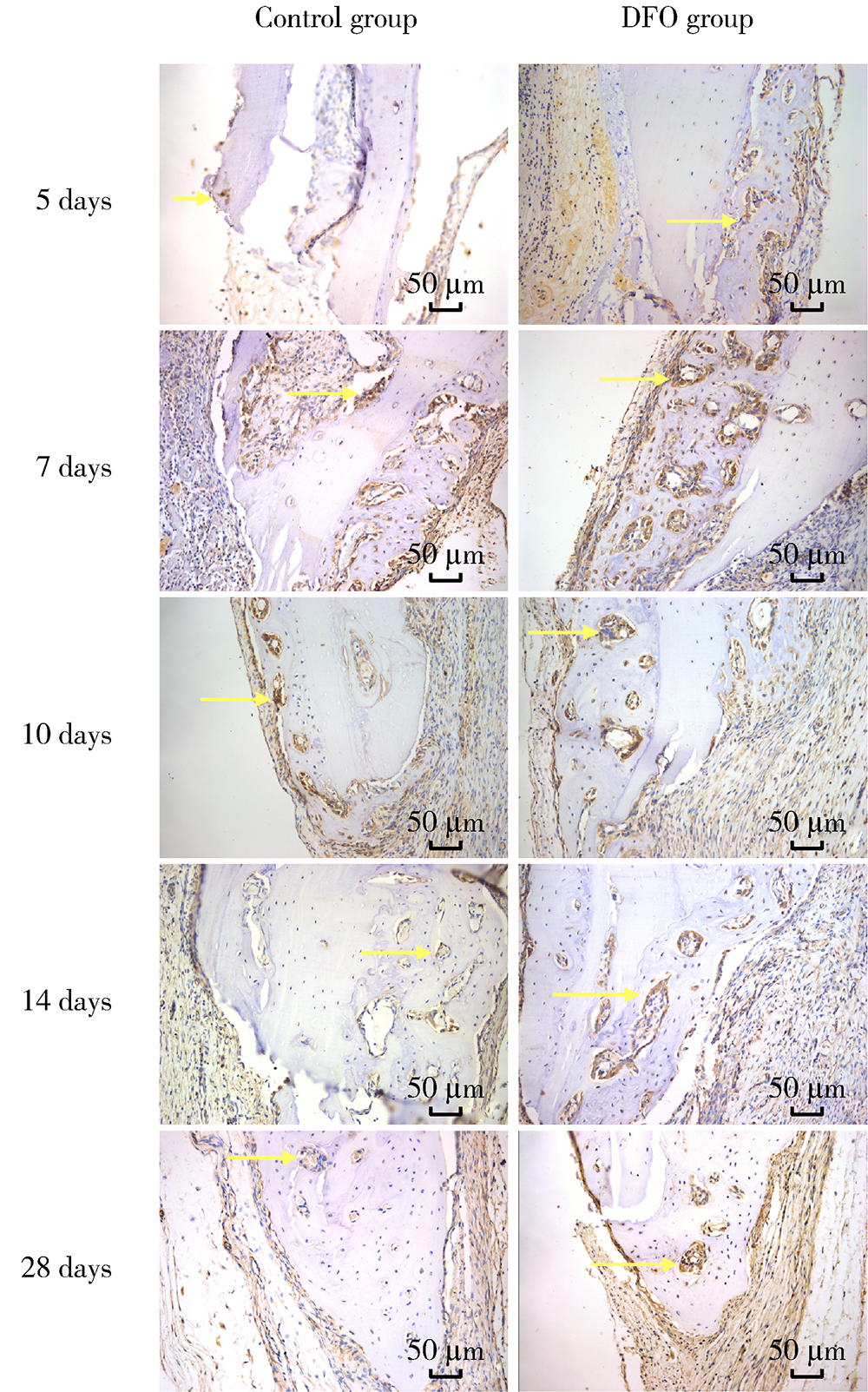
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (6): 1171-1177. doi: 10.19723/j.issn.1671-167X.2021.06.027
Previous Articles Next Articles
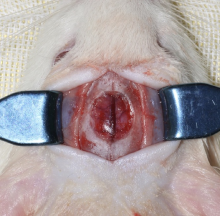
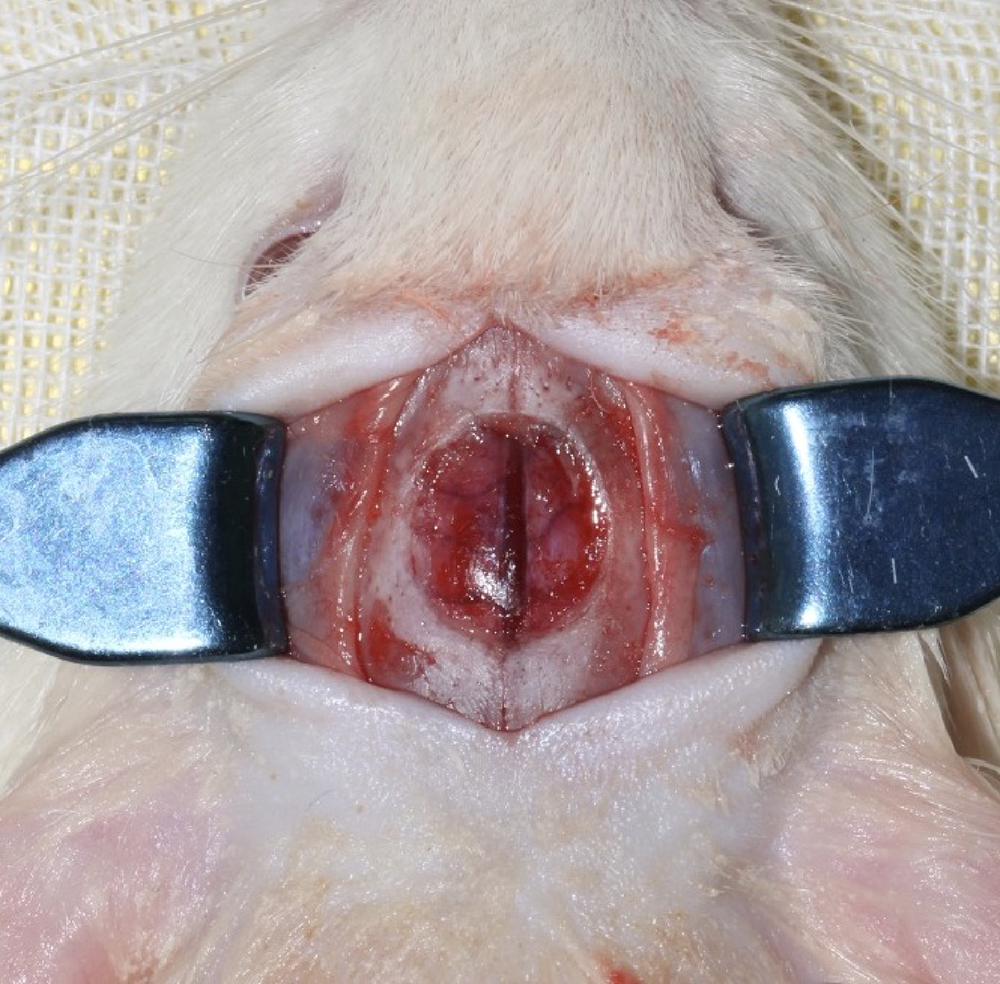
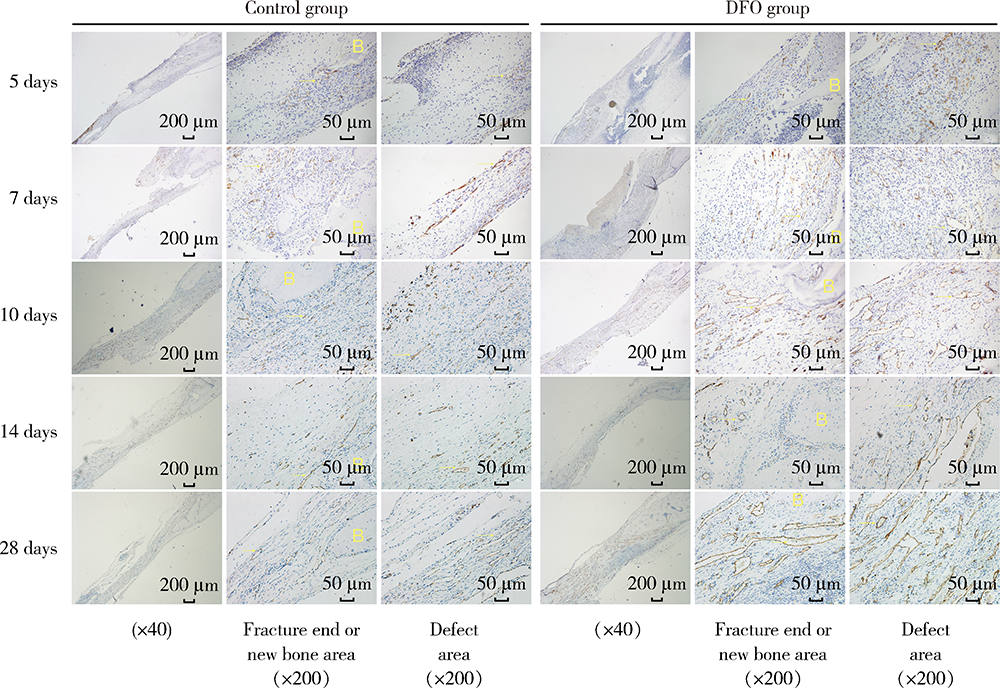
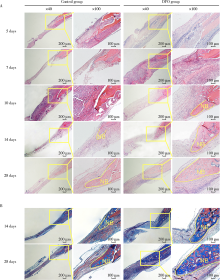
Early constant observation of the effect of deferoxamine mesylate on improvement of vascularized bone regeneration in SD rat skull critical size defect model
DU Wen-yu,YANG Jing-wen( ),JIANG Ting(
),JIANG Ting( )
)
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing 100081, China
CLC Number:
- R318.08
| [1] |
Yellowley CE, Genetos DC. Hypoxia signaling in the skeleton: Implications for bone health[J]. Curr Osteoporos Rep, 2019, 17(1):26-35.
doi: 10.1007/s11914-019-00500-6 pmid: 30725321 |
| [2] |
Holden P, Nair L. Deferoxamine: An angiogenic and antioxidant molecule for tissue regeneration[J]. Tissue Eng Part B Rev, 2019, 25(6):461-470.
doi: 10.1089/ten.teb.2019.0111 |
| [3] |
Temiz G, Sirinoglu H, Yesiloglu N, et al. Effects of deferoxamine on fat graft survival[J]. Facial Plast Surg, 2016, 32(4):438-443.
doi: 10.1055/s-0036-1584236 |
| [4] | Farzan R, Moeinian M, Abdollahi A, et al. Effects of amniotic membrane extract and deferoxamine on angiogenesis in wound healing: An in vivo model[J]. J Wound Care, 2018, 27(Suppl 6):s26-s32. |
| [5] |
Ram M, Singh V, Kumawat S, et al. Deferoxamine modulates cytokines and growth factors to accelerate cutaneous wound healing in diabetic rats[J]. Eur J Pharmacol, 2015, 764:9-21.
doi: 10.1016/j.ejphar.2015.06.029 |
| [6] |
Gao SQ, Chang C, Li JJ, et al. Co-delivery of deferoxamine and hydroxysafflor yellow A to accelerate diabetic wound healing via enhanced angiogenesis[J]. Drug Deliv, 2018, 25(1):1779-1789.
doi: 10.1080/10717544.2018.1513608 |
| [7] |
Yang Q, He GW, Underwood MJ, et al. Cellular and molecular mechanisms of endothelial ischemia/reperfusion injury: Perspectives and implications for postischemic myocardial protection[J]. Am J Transl Res, 2016, 8(2):765-777.
pmid: 27158368 |
| [8] |
Jia P, Chen H, Kang H, et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis[J]. J Biomed Mater Res A, 2016, 104(10):2515-2527.
doi: 10.1002/jbm.a.35793 |
| [9] | 姚洋, 杜宇, 古霞, 等. 局部注射外源性神经生长因子促进小鼠钛种植体周骨胶原早期成熟的研究[J]. 华西口腔医学杂志, 2018, 36(2):128-132. |
| [10] |
Koivunen P, Serpi R, Dimova EY. Hypoxia-inducible factor prolyl 4-hydroxylase inhibition in cardiometabolic diseases[J]. Pharmacol Res, 2016, 114:265-273.
doi: S1043-6618(16)31160-4 pmid: 27832958 |
| [11] | Lanigan S, Corcoran AE, Wall A, et al. Acute hypoxic exposure and prolyl-hydroxylase inhibition improves synaptic transmission recovery time from a subsequent hypoxic insult in rat hippocampus[J]. Brain Res, 2018, 1701:212-218. |
| [12] |
Farberg AS, Sarhaddi D, Donneys A, et al. Deferoxamine enhances bone regeneration in mandibular distraction osteogenesis[J]. Plast Reconstr Surg, 2014, 133(3):666-671.
doi: 10.1097/01.prs.0000438050.36881.a9 pmid: 24572857 |
| [13] |
Zhang J, Zheng L, Wang Z, et al. Lowering iron level protects against bone loss in focally irradiated and contralateral femurs through distinct mechanisms[J]. Bone, 2019, 120:50-60.
doi: S8756-3282(18)30369-7 pmid: 30304704 |
| [14] |
Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions[J]. Nat Rev Rheumatol, 2015, 11(1):45-54.
doi: 10.1038/nrrheum.2014.164 pmid: 25266456 |
| [15] | Morgan EF, Giacomo A, Gerstenfeld LC. Overview of skeletal repair (fracture healing and its assessment)[J]. Methods Mol Biol, 2021, 2230:17-37. |
| [16] |
Donneys A, Deshpande SS, Tchanque-Fossuo CN, et al. Deferoxa-mine expedites consolidation during mandibular distraction osteogenesis[J]. Bone, 2013, 55(2):384-390.
doi: 10.1016/j.bone.2013.04.005 pmid: 23598047 |
| [17] | Zimna A, Kurpisz M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: Applications and therapies[J]. Biomed Res Int, 2015, 2015:549412. |
| [18] |
Drager J, Harvey EJ, Barralet J. Hypoxia signalling manipulation for bone regeneration[J]. Expert Rev Mol Med, 2015, 17:e6.
doi: 10.1017/erm.2015.4 |
| [19] |
Matsubara H, Hogan DE, Morgan EF, et al. Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis[J]. Bone, 2012, 51(1):168-180.
doi: 10.1016/j.bone.2012.02.017 pmid: 22391215 |
| [20] |
Bouletreau PJ, Warren SM, Spector JA, et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: Implications for fracture healing[J]. Plast Reconstr Surg, 2002, 109(7):2384-2397.
pmid: 12045566 |
| [1] | Yun-yi XU,Zheng-zheng SU,Lin-mao ZHENG,Meng-ni ZHANG,Jun-ya TAN,Ya-lan YANG,Meng-xin ZHANG,Miao XU,Ni CHEN,Xue-qin CHEN,Qiao ZHOU. Read-through circular RNA rt-circ-HS promotes hypoxia inducible factor 1α expression and renal carcinoma cell proliferation, migration and invasiveness [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 217-227. |
| [2] | WANG Jing-qi,WANG Xiao. In vivo study of strontium-doped calcium phosphate cement for biological properties [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 378-383. |
| [3] | ZHANG Sheng-nan,AN Na,OUYANG Xiang-ying,LIU Ying-jun,WANG Xue-kui. Role of growth arrest-specific protein 6 in migration and osteogenic differentiation of human periodontal ligament cells [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 9-15. |
| [4] | Mei WANG, Bo-wen LI, Si-wen WANG, Yu-hua LIU. Preparation and osteogenic effect study of small intestinal submucosa sponge [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 952-958. |
| [5] | Ying CHEN,Zhong-ning LIU,Bo LI,Ting JIANG. Preparation of aspirin sustained-release microsphere and its in vitro releasing [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 907-912. |
| [6] | SUI Hua-xin, LV Pei-jun, WANG Yong, FENG Yu-chi. Effects of low level laser irradiation on the osteogenic capacity of sodium alginate/gelatin/human adipose-derived stem cells 3D bio-printing construct [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 868-875. |
| [7] | LU Jin-hui, QIAN Jun, LIU He, ZHU Jun-xia. Clinical study on autologus platelet-rich fibrin aided revascularization of immature permanent teeth [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 672-679. |
| [8] | LIU Jing-yin, CHEN Fei, GE Yan-jun, WEI Ling, PAN Shao-xia, FENG Hai-lan. Influence of implants prepared by selective laser melting on early bone healing [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 117-122. |
| [9] | ZHANG Lu-feng, LING Yun-peng, YANG Hang, GONG Yi-chen, SONG Zhi-ming, WAN Feng. Comparison of outcomes of two minimally invasive approaches for multi-vessel coronary revascularization [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 1066-1070. |
| [10] | CHEN Fei, PAN Shao-xia, FENG Hai-lan. Distribution and content of transforming growth factor-β1 and vascular endothelial growth factor in each layer of concentrated growth factors [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 860-865. |
| [11] | QIN Xue-yan, ZHAO Hua-xiang, ZHANG Qian, CHEN Feng, LIN Jiu-xiang. NELL-1: a novel highly efficient and specific growth factor [J]. Journal of Peking University(Health Sciences), 2016, 48(2): 380-383. |
| [12] | GE Wen-shu, TANG Yi-man, ZHANG Xiao, LIU Yun-song, ZHOU Yong-sheng. Establishing a luciferase reporter system to evaluate osteogenic differentiation potential of human adipose-derived stem cells [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 170-174. |
| [13] | SONG Yang, WANG Xiao-fei, WANG Yu-guang, SUN Yu-chun, LV Pei-jun. Osteogenesis of human adipose-derived mesenchymal stem cells-biomaterial mixture in vivo after 3D bio-printing [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 45-50. |
| [14] | 欧Meng-恩 , ZHANG Xiao, LIU Yun-Song, GE Yan-Jun, ZHOU Yong-Sheng. Ectopic osteogenesis of stromal cell-derived factor 1 combined with simvastatin loaded collagen scaffold in vivo [J]. Journal of Peking University(Health Sciences), 2015, 47(1): 47-51. |
| [15] | DING Qian, ZHANG Feng-qiu, MA Yu-shi. Effect of icariin on osteoblastic differentiation gene expression of human periodontal ligament cells [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 975-978. |
|
||