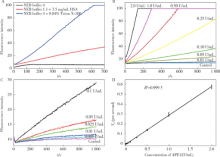
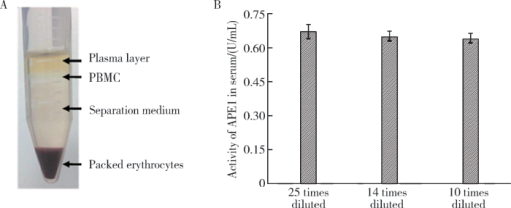
Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (3): 487-492. doi: 10.19723/j.issn.1671-167X.2019.03.016
Previous Articles Next Articles
Fluorescence assay for the detection of apurinic/apyrimidinic endonuclease 1 (APE1) activity in human blood samples
- Beijing National Laboratory for Molecular Sciences, MOE Key Laboratory of Bioorganic Chemistry and Molecular Engineering, College of Chemistry and Molecular Engineering, Peking University, Beijing 100871, China
CLC Number:
- R446.1
| [1] |
Li M , Wilson DM 3rd. Human apurinic/apyrimidinic endo-nuclease 1[J]. Antioxid Redox Signal, 2014,20(4):678-707.
doi: 10.1089/ars.2013.5492 |
| [2] |
Whitaker AM, Freudenthal BD . APE1: A skilled nucleic acid surgeon[J]. DNA Repair (Amst), 2018,71:93-100.
doi: 10.1016/j.dnarep.2018.08.012 |
| [3] |
Traversi D, Cervella P, Gilli G . Evaluating the genotoxicity of urban PM2.5 using PCR-based methods in human lung cells and the Salmonella TA98 reverse test[J]. Environ Sci Pollut Res, 2015,22(2):1279-1289.
doi: 10.1007/s11356-014-3435-1 |
| [4] |
Simeonov A, Kulkarni A, Dorjsuren D , et al. Identification and characterization of inhibitors of human apurinic/apyrimidinic endonuclease APE1[J]. PLoS One, 2009,4(6):e5740.
doi: 10.1371/journal.pone.0005740 |
| [5] |
Han J, Zhuo Y, Chai Y , et al. Ultrasensitive electrochemical strategy for trace detection of APE-1 via triple signal amplification strategy[J]. Biosens Bioelectron, 2013,41:116-122.
doi: 10.1016/j.bios.2012.07.082 |
| [6] |
Kreklau EL, Limp-Foster M, Liu N , et al. A novel fluorometric oligonucleotide assay to measure O 6-methylguanine DNA methyltransferase, methylpurine DNA glycosylase, 8-oxoguanine DNA glycosylase and abasic endonuclease activities: DNA repair status in human breast carcinoma cells overexpressing methylpurine DNA glycosylase [J]. Nucleic Acids Res, 2001,29(12):2558-2566.
doi: 10.1093/nar/29.12.2558 |
| [7] | Zhang S, He L, Dai N , et al. Serum APE1 as a predictive marker for platinum-based chemotherapy of non-small cell lung cancer patients[J]. Oncotarget, 2016,7(47):77482-77494. |
| [8] |
Kirkali G, Jaruga P, Reddy P . Identification and quantification of DNA repair protein apurinic/apyrimidinic endonuclease 1 (APE1) in human cells by liquid chromatography/isotope-dilution tandem mass spectrometry[J]. PLoS One, 2013,8(7):e69894.
doi: 10.1371/journal.pone.0069894 |
| [9] |
Coskun E, Jaruga P, Reddy P . Extreme expression of DNA repair protein apurinic/apyrimidinic endonuclease 1 (APE1) in human breast cancer as measured by liquid chromatography and isotope dilution tandem mass spectrometry[J]. Biochemistry, 2015,54(38):5787-5790.
doi: 10.1021/acs.biochem.5b00928 |
| [10] |
Fang S, Chen L, Zhao M . Unimolecular chemically modified DNA fluorescent probe for one-step quantitative measurement of the activity of human apurinic/apyrimidinic endonuclease 1 in biological samples[J]. Anal Chem, 2015,87(24):11952-11956.
doi: 10.1021/acs.analchem.5b03939 |
| [11] |
Greening DW, Simpson RJ . Characterization of the low-molecular-weight human plasma peptidome[J]. Methods Mol Biol, 2017,1619:63-79.
doi: 10.1007/978-1-4939-7057-5 |
| [12] |
Wang D, Zhuo JQ, Zhao MP . A simple and rapid competitive enzyme-linked immunosorbent assay (cELISA) for high-throughput measurement of secretory immunoglobulin A (sIgA) in saliva[J]. Talanta, 2010,82(1):432-436.
doi: 10.1016/j.talanta.2010.04.040 |
| [13] |
Li XJ, Mei X, Huang YF , et al. Simple label-free fluorescence detection of apurinic/apyrimidinic endonuclease 1 activity and its inhibitor using the abasic site-binding fluorophore[J]. Anal Methods, 2019,11(6):739-743.
doi: 10.1039/C8AY02633E |
| [14] |
Evans AR, Limp-Foster M, Kelley MR . Going APE over ref-1[J]. Mutat Res, 2000,461(2):83-108.
doi: 10.1016/S0921-8777(00)00046-X |
| [15] |
Antoniali G, Malfatti MC, Tell G . Unveiling the non-repair face of the base excision repair pathway in RNA processing: A missing link between DNA repair and gene expression?[J]. DNA Repair, 2017,56:65-74.
doi: 10.1016/j.dnarep.2017.06.008 |
| [16] | Kelley MR, Cheng L, Foster R , et al. Elevated and altered expression of the multifunctional DNA base excision repair and redox enzyme Ape1/ref-1 in prostate cancer[J]. Clin Cancer Res, 2001,7(4):824-830. |
| [17] |
Wang D, Xiang DB, Yang XQ , et al. APE1 overexpression is associated with cisplatin resistance in non-small cell lung cancer and targeted inhibition of APE1 enhances the activity of cisplatin in A549 cells[J]. Lung Cancer, 2009,66(3):298-304.
doi: 10.1016/j.lungcan.2009.02.019 |
| [18] | Moore DH, Michael H, Tritt R , et al. Alterations in the expression of the DNA repair/redox enzyme APE/ref-1 in epithelial ovarian cancers[J]. Clin Cancer Res, 2000,6(2):602-609. |
| [19] |
Shin JH, Choi S, Lee YR , et al. APE1/Ref-1 as a serological biomarker for the detection of bladder cancer[J]. Cancer Res Treat, 2015,47(4):823-833.
doi: 10.4143/crt.2014.074 |
| [20] |
Dai N, Cao XJ, Li MX , et al. Serum APE1 autoantibodies: a novel potential tumor marker and predictor of chemotherapeutic efficacy in non-small cell lung cancer[J]. PLoS One, 2013,8(3):e58001.
doi: 10.1371/journal.pone.0058001 |
| [1] | Yu-xiao WU,Yi-fan KANG,Qian-ying MAO,Zi-meng LI,Xiao-feng SHAN,Zhi-gang CAI. Application of methylene blue near-infrared fluorescence imaging for oral sentinel lymph node mapping in rats [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 684-688. |
| [2] | Wei WANG,Xin LI,Ping LIU,Ying DONG. Clinical value of fluorescence in situ hybridization with MDM2 and DDIT3 probe in diagnosis of liposarcoma [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 228-233. |
| [3] | Xue-ping WANG,Yu-ya-nan ZHANG,Tian-lan LU,Zhe LU,Zhe-wei KANG,Yao-yao SUN,Wei-hua YUE. Variations in fecal microbiota of first episode schizophrenia associated with clinical assessment and serum metabolomics [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 863-873. |
| [4] | LI Zheng,WANG Xiao,HONG Tian-pei,WANG Hao-jie,GAO Zhan-yi,WAN Meng. Mechanism of advanced glycation end products inhibiting the proliferation of peripheral blood mononuclear cells and osteoblasts in rats [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 355-363. |
| [5] | CHI Yan-ting,ZHANG Yan-ping,ZHANG Qiu-lu,LIU Cui-ling,LI bin-bin. Clinicopathological analysis of mucosa associated lymphoid tissue lymphoma secondary to Sjögren’s syndrome in salivary gland [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 40-45. |
| [6] | Bing-wei HUANG,Jie WANG,Peng ZHANG,Zhe LI,Si-cheng BI,Qiang WANG,Cai-bo YUE,Kun-lin YANG,Xue-song LI,Li-qun ZHOU. Application of indocyanine green in complex upper urinary tract repair surgery [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 651-656. |
| [7] | Shu-dong ZHANG,Peng HONG,Bin-shuai WANG,Shao-hui DENG,Fan ZHANG,Li-yuan TAO,Cai-guang CAO,Zhen-hua HU,Lu-lin MA. Usefulness of the indocyanine green fluorescence imaging technique in laparoscopic partial nephrectomy [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 657-662. |
| [8] | WU Peng-hui, XIE Yao, ZHAO Wei-hong, HUA Ying, SUN Qing, LI Shuo, WU Ye, LU Xin-tian. Clinical characteristics analysis of children with reversible posterior leukoen-cephalopathy syndrome during the treatment of hematological tumor [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 662-665. |
| [9] | FENG Yong-liang, FAN Jing-hui, LIN Xian-juan, YANG Ji-chun, CUI Qing-hua, TANG Xin-jing, XU Guo-heng, GENG Bin. Facilitating the measurement of circulatory hydrogen sulfide with fluorescence probe-coated microplates [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 1060-1065. |
| [10] | ZHANG Yi-lan, WANG Zhi-feng, CHEN Ning. Expression of serum IgG4 in patients with different diseases [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 961-964. |
| [11] | DENG Hui, MA Jun-fan, JING Zi-yang, LIANG Yao-xian, A La-ta, LIU Yang, QIU Xiao-yan, WANG Yue. Primary investigation of immunoglobulin A synthesis and secretion in human mesangial cells [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 948-953. |
| [12] | YU Jian-feng, JIN Yue-bo, HE Jing, AN Yuan, LI Zhan-guo. Changes of serum Krebs von den Lungen-6 levels in interstitial lung disease associated with dermatomyositis and secondary Sjögren’s syndrome: a case report [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 910-914. |
| [13] | WANG Fang, ZHANG Yan-qin, DING Jie, YU Li-xia. Detection of large deletions in X linked Alport syndrome using competitive multiplex fluorescence polymerase chain reaction [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 760-767. |
| [14] | LIU Jia-yuan, PENG Xiang, NING Xiang-hui, LI Teng, PENG Shuang-he, WANG Jiang-yi, LIU Sheng-jie, DING Yi, CAI Lin, GONG Kan. Clinical value of fluorescence in situ hybridization positive of exfoliated urothelial cells in urothelial carcinoma [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 585-589. |
| [15] | CHEN Zhi-qiang, WANG Ying, MI Xian-jun, CHEN Ang, HUANG Hua-yong, ZHONG Shou-jun, DENG Wen-tong, LIU Chao-fan, XU Xiu-mei, DAI Xin-zhen. Comparison between poly hydroxy acrylic acid and Van-clear replacing the tradi-tional reagents to detect the cervical hTERC genes by adopting FISH technique [J]. Journal of Peking University(Health Sciences), 2016, 48(2): 356-360. |
|
||