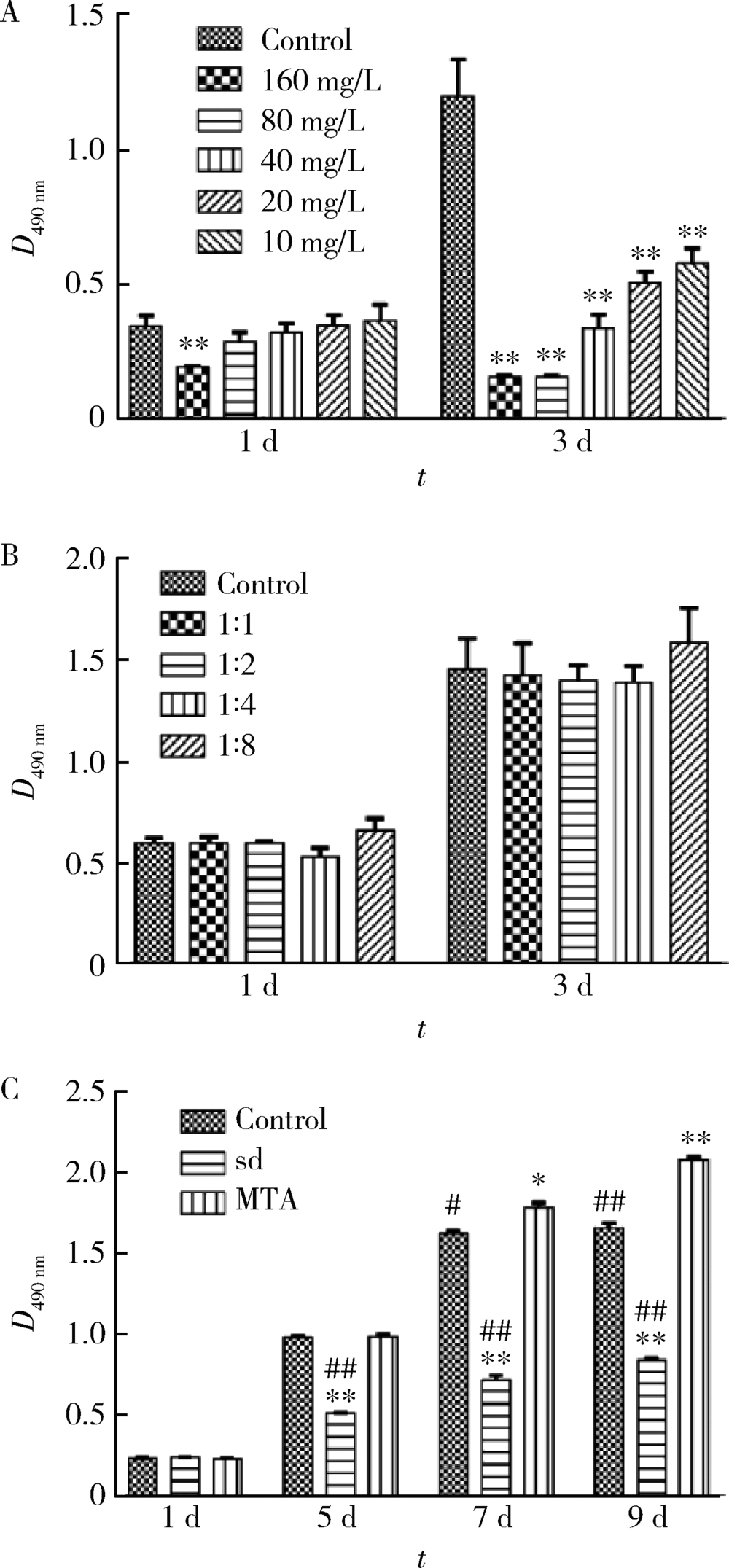
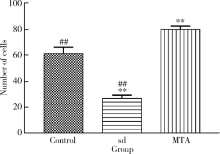
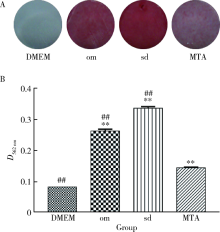
Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (6): 1108-1114. doi: 10.19723/j.issn.1671-167X.2019.06.023
Previous Articles Next Articles
Effects of mineral trioxide aggregate and ethanolic extracts of Shandong propolis on the biological properties of human dental pulp fibroblasts
Bing-qing SHI,Xiao-jing YUAN,Yu-ming ZHAO( )
)
- Department of Pediatric Dentistry, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R788.2
| [1] | Al-Shaher A, Wallace J, Agarwal S , et al. Effect of propolis on human fibroblasts from the pulp and periodontal ligament[J]. J Endod, 2004,30(5):359-361. |
| [2] | Al-Haj Ali SN . In vitro toxicity of propolis in comparison with other primary teeth pulpotomy agents on human fibroblasts[J]. J Investig Clin Dent, 2016,7(3):308-313. |
| [3] | Jacob A, Parolia A, Pau A , et al. The effects of Malaysian propolis and Brazilian red propolis on connective tissue fibroblasts in the wound healing process[J]. BMC Complement Altern Med, 2015,15(1):294. |
| [4] | Moradi S, Saghravanian N, Moushekhian S , et al. Immunohistochemical evaluation of fibronectin and tenascin following direct pulp capping with mineral trioxide aggregate, platelet-rich plasma and propolis in dogs’ teeth[J]. Iran Endod J, 2015,10(3):188-192. |
| [5] | Parolia A, Kundabala M, Rao N , et al. A comparative histological analysis of human pulp following direct pulp capping with Propolis, mineral trioxide aggregate and Dycal[J]. Aust Dent J, 2010,55(1):59-64. |
| [6] | ISO10993-12-2007医疗器械生物学评价——第12部分:样品制备与参照样品[S]. |
| [7] | Rodrigues EM, Cornélio ALG, Mestieri LB , et al. Human dental pulp cells response to MTA and MTA Plus: Cytotoxicity and gene expression analysis[J]. International Endodontic Journal, 2016,50(8):780-789. |
| [8] | Zare Jahromi M, Ranjbarian P, Shiravi S . Cytotoxicity evaluation of Iranian propolis and calcium hydroxide on dental pulp fibroblasts[J]. J Dent Res Dent Clin Dent Prospects, 2014,8(3):130-133. |
| [9] | 刘颖婷, 张玉皓 . 水溶蜂胶对人牙髓成纤维细胞的毒性作用研究[J]. 中国现代医学杂志, 2015,25(30):18-22. |
| [10] | Chang H, Wang Y, Yin X , et al. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy[J]. BMC Complement Altern Med, 2017,17(1):471. |
| [11] | Margunato S, Tasli PN, Aydin S , et al. In vitro evaluation of ProRoot MTA, Biodentine, and MM-MTA on human alveolar bone marrow stem cells in terms of biocompatibility and mineralization[J]. J Endod, 2015,41(10):1646-1652. |
| [12] | Kabala-Dzik A, Rzepecka-Stojko A, Kubina R , et al. Migration rate inhibition of breast cancer cells treated by caffeic acid and caffeic acid phenethyl ester: An in vitro comparison study[J]. Nutrients, 2017,9(10):1144. |
| [13] | Bueno-Silva B, Franchin M, Alves C , et al. Main pathways of action of Brazilian red propolis on the modulation of neutrophils migration in the inflammatory process[J]. Phytomedicine, 2016,23(13):1583-1590. |
| [14] | Ahangari Z, Naseri M, Jalili M , et al. Effect of propolis on dentin regeneration and the potential role of dental pulp stem cell in Guinea pigs[J]. Cell J, 2012,13(4):223-228. |
| [15] | Lima Cavendish R, de Souza Santos J, Belo Neto R , et al. Anti-nociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents[J]. J Ethnopharmacol, 2015,173:127-133. |
| [16] | das Neves MV, da Silva TM, Lima Ede O , et al. Isoflavone for-mononetin from red propolis acts as a fungicide against Candida sp.[J]. Braz J Microbiol, 2016,47(1):159-166. |
| [17] | Gemiarto AT, Ninyio NN, Lee SW , et al. Isoprenyl caffeate, a major compound in manuka propolis, is a quorum-sensing inhibitor in Chromobacterium violaceum[J]. Antonie Van Leeuwenhoek, 2015,108(2):491-504. |
| [18] | Saha S, Nair R and Asrani H. Comparative evaluation of propolis, metronidazole with chlorhexidine, calcium hydroxide and curcuma longa extract as intracanal medicament against E. faecalis: An in vitro study [J]. J Clin Diagn Res, 2015, 9(11): ZC19-21. |
| [19] | Bhandari S, Ashwini TS, Patil CR . An in vitro evaluation of antimicrobial efficacy of 2% chlorhexidine gel,propolis and calcium hydroxide against Enterococcus faecalis in human root dentin [J]. J Clin Diagn Res, 2014, 8(11): ZC60-63. |
| [20] | Neiva K, Catalfamo D, Holliday S , et al. Propolis decreases lipopolysaccharide-induced inflammatory mediators in pulp cells and osteoclasts[J]. Dent Traumatol, 2014,30(5):362-367. |
| [21] | Kusum B, Rakesh K, Richa K . Clinical and radiographical evaluation of mineral trioxide aggregate, biodentine and propolis as pulpotomy medicaments in primary teeth[J]. Restor Dent Endod, 2015,40(4):276-285. |
| [22] | Altunsoy M, Tanrıver M, Türkan U , et al. In vitro evaluation of microleakage and microhardness of ethanolic extracts of propolis in different proportions added to glass ionomer cement[J]. J Clin Pediatr Dent, 2016,40(2):136-140. |
| [23] | Prabhakar AR, Balehosur DV, Basappa N. Comparative evaluation of shear bond strength and fluoride release of conventional glass ionomer with 1% ethanolic extract of propolis incorporated glass ionomer cement: In vitro study [J]. J Clin Diagn Res, 2016, 10(5): ZC88-91. |
| [24] | Grenho L, Barros J, Ferreira C , et al. In vitro antimicrobial acti-vity and biocompatibility of propolis containing nanohydroxyapatite[J]. Biomed Mater, 2015,10(2):025004. |
| [1] | Shan HE,Xin CHEN,Qi CHENG,Lingjiang ZHU,Peiyu ZHANG,Shuting TONG,Jing XUE,Yan DU. Tofacitinib inhibits the transformation of lung fibroblasts into myofibroblasts through JAK/STAT3 pathway [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 505-511. |
| [2] | Yu-yang YE,Lin YUE,Xiao-ying ZOU,Xiao-yan WANG. Characteristics and microRNA expression profile of exosomes derived from odontogenic dental pulp stem cells [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 689-696. |
| [3] | TIAN Jing,QIN Man,CHEN Jie,XIA Bin. Early loss of primary molar and permanent tooth germ caused by the use of devitalizer during primary molar root canal therapy: Two cases report [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 381-385. |
| [4] | QIAN Kun,PAN Jie,ZHU Wen-hao,ZHAO Xiao-yi,LIU Chang,YONG Wei. Evaluation of bioceramic putty repairmen iRoot and mineral trioxide aggregate in mature permanent teeth pulpotomy [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 113-118. |
| [5] | GAO Hong-yu,MENG Huan-xin,HOU Jian-xia,HUANG Bao-xin,LI Wei. Expression and distribution of calprotectin in healthy and inflamed periodontal tissues [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 744-749. |
| [6] | Xiao-min GAO,Xiao-ying ZOU,Lin YUE. Mediated pathways of exosomes uptake by stem cells of apical papilla [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 43-50. |
| [7] | Jing-yi LI,Sai-nan WANG,Yan-mei DONG. Anti-inflammatory and repaired effects of non-steroidal anti-inflammatory drugs on human dental pulp cells [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 24-29. |
| [8] | Jing XIE,Yu-ming ZHAO,Nan-quan RAO,Xiao-tong WANG,Teng-jiao-zi FANG,Xiao-xia LI,Yue ZHAI,Jing-zhi LI,Li-hong GE,Yuan-yuan WANG. Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 900-906. |
| [9] | Miao ZHENG,Ling-lu ZHAN,Zhi-qiang LIU,He-ping LI,Jian-guo TAN. Effect of different plasma treated zirconia on the adhensive behaviour of human gingival fibroblasts [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 315-320. |
| [10] | LI Shuang, ZHANG Qing. Effect of smear layer on apical sealing ability of mineral trioxide aggregate (MTA) Plus through the sucrose penetration mode [J]. Journal of Peking University(Health Sciences), 2018, 50(3): 560-563. |
| [11] | DONG Ying-tao, TIAN Fu-cong, JIA Bin, ZU Bin, WANG Xiao-yan. Influence of setting time on bond strength of different bioactive pulp capping mate-rials with dental adhesive [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 58-62. |
| [12] | JIA Wei-qian, ZHAO Yu-ming, GE Li-hong. Recombinant human transforming growth factor β1 promotes dental pulp stem cells proliferation and mineralization [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 680-681. |
| [13] | LIU Yi, WANG Sai-nan, CUI Cai-yun, DONG Yan-mei. Influence of the Arg-Gly-Asp-Ser sequence on the biological effects of bioactive glass on human dental pulp cells [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 326-330. |
| [14] | WEN Quan, ZHAO Yu-ming, WANG Yuan-yuan, WANG Xu, LING Long, GE Li-hong. Effects of stromal cell-derived factor-1 on proliferation, migration, and odontoblastic differentiation of human dental pulp stem cells [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 23-29. |
| [15] | YU Tao, JIANG Ting, WEI Qing-mei, LI Yi-fen, David L. Kaplan. Wound healing effects of silk fibroin-bone morphogenetic protein-2 scaffolds on inflammatory pulp in rats [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 814-819. |
|
||