Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (5): 863-873. doi: 10.19723/j.issn.1671-167X.2022.05.014
Previous Articles Next Articles
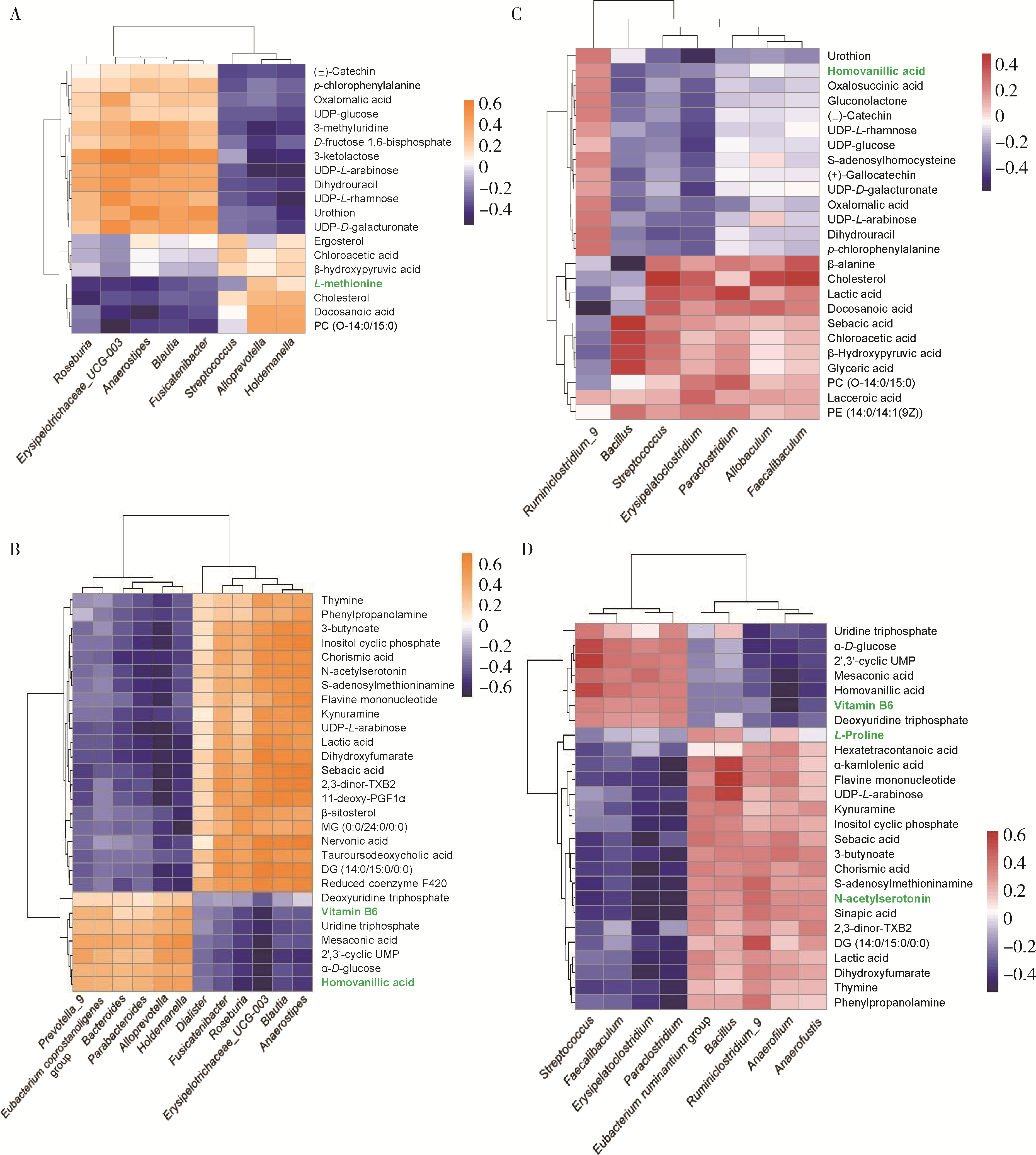
Variations in fecal microbiota of first episode schizophrenia associated with clinical assessment and serum metabolomics
Xue-ping WANG,Yu-ya-nan ZHANG,Tian-lan LU,Zhe LU,Zhe-wei KANG,Yao-yao SUN,Wei-hua YUE*( )
)
- Peking University Sixth Hospital, Peking University Institute of Mental Health, NHC Key Laboratory of Mental Health (Peking University), National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Beijing 100191, China
CLC Number:
- R749.3
| 1 |
Samochowiec J , Misiak B . Gut microbiota and microbiome in schizophrenia[J]. Curr Opin Psychiatry, 2021, 34 (5): 503- 507.
doi: 10.1097/YCO.0000000000000733 |
| 2 |
Eckburg PB , Bik EM , Bernstein CN , et al. Diversity of the human intestinal microbial flora[J]. Science, 2005, 308 (5728): 1635- 1638.
doi: 10.1126/science.1110591 |
| 3 |
Gill SR , Pop M , Deboy RT , et al. Metagenomic analysis of the human distal gut microbiome[J]. Science, 2006, 312 (5778): 1355- 1359.
doi: 10.1126/science.1124234 |
| 4 |
Collins SM , Surette M , Bercik P . The interplay between the intestinal microbiota and the brain[J]. Nat Rev Microbiol, 2012, 10 (11): 735- 742.
doi: 10.1038/nrmicro2876 |
| 5 |
Kanji S , Fonseka TM , Marshe VS , et al. The microbiome-gut-brain axis: Implications for schizophrenia and antipsychotic induced weight gain[J]. Eur Arch Psychiatry Clin Neurosci, 2018, 268 (1): 3- 15.
doi: 10.1007/s00406-017-0820-z |
| 6 |
Arneth BM . Gut-brain axis biochemical signalling from the gastrointestinal tract to the central nervous system: gut dysbiosis and altered brain function[J]. Postgrad Med J, 2018, 94 (1114): 446- 452.
doi: 10.1136/postgradmedj-2017-135424 |
| 7 |
Thaker GK , Carpenter WT . Advances in schizophrenia[J]. Nat Med, 2001, 7 (6): 667- 671.
doi: 10.1038/89040 |
| 8 | He Y, Kosciolek T, Tang J, et al. Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis[J/OL]. Eur Psychiatry, 2018, 53: 37-45(2018-06-02)[2022-06-01]. https://linkinghub.elsevier.com/retrieve/pii/S0924-9338(18)30106-8. |
| 9 | Ma X, Asif H, Dai L, et al. Alteration of the gut microbiome in first-episode drug-naive and chronic medicated schizophrenia correlate with regional brain volumes[J/OL]. J Psychiatr Res, 2020, 123: 136-144(2020-02-08)[2022-06-01]. https://linkinghub.elsevier.com/retrieve/pii/S0022-3956(19)31162-8. |
| 10 | Nguyen TT, Kosciolek T, Maldonado Y, et al. Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects[J/OL]. Schizophr Res, 2019, 204: 23-29(2018-09-26)[2022-06-01]. https://linkinghub.elsevier.com/retrieve/pii/S0920-9964(18)30572-3. |
| 11 | Schwarz E, Maukonen J, Hyytiainen T, et al. Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response[J/OL]. Schizophr Res, 2018, 192: 398-403[2022-06-01]. https://linkinghub.elsevier.com/retrieve/pii/S0920-9964(17)30204-9. |
| 12 | Yuan X, Zhang P, Wang Y, et al. Changes in metabolism and microbiota after 24-week risperidone treatment in drug naive, normal weight patients with first episode schizophrenia[J/OL]. Schizophr Res, 2018, 201: 299-306(2018-05-30)[2022-06-01]. https://linkinghub.elsevier.com/retrieve/pii/S0920-9964(18)30274-3. |
| 13 |
Nicholson JK , Holmes E , Kinross J , et al. Host-gut microbiota metabolic interactions[J]. Science, 2012, 336 (6086): 1262- 1267.
doi: 10.1126/science.1223813 |
| 14 | Vernocchi P, Chierico F, Putignani L. Gut microbiota profiling: Metabolomics based approach to unravel compounds affecting human health[J/OL]. Front Microbiol, 2016, 7: 1144(2016-07-26)[2022-06-01]. https://www.frontiersin.org/articles/10.3389/fmicb.2016.01144/full |
| 15 | Swann JR , Want EJ , Geier FM , et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments[J]. Proc Natl Acad Sci USA, 2011, 108 (Suppl 1): 4523- 4530. |
| 16 |
Zhu F , Guo R , Wang W , et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice[J]. Mol Psychiatry, 2020, 25 (11): 2905- 2918.
doi: 10.1038/s41380-019-0475-4 |
| 17 | Shen Y, Xu J, Li Z, et al. Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study[J/OL]. Schizophr Res, 2018, 197: 470-477(2018-01-17)[2022-06-01]. https://linkinghub.elsevier.com/retrieve/pii/S0920-9964(18)30002-1. |
| 18 | Reiterer M , Schmidt-Kastner R , Milton SL . Methionine sulfoxide reductase (Msr) dysfunction in human brain disease[J]. Free radical research, 2019, 53 (11/12): 1144- 1154. |
| 19 | Wang L , Alachkar A , Sanathara N , et al. A Methionine-induced animal model of schizophrenia: Face and predictive validity[J]. Int J Neuropsychopharmacol, 2015, 18 (12): 1- 11. |
| 20 |
Crabtree GW , Park AJ , Gordon JA , et al. Cytosolic accumulation of L-proline disrupts GABA-ergic transmission through GAD blockade[J]. Cell Rep, 2016, 17 (2): 570- 582.
doi: 10.1016/j.celrep.2016.09.029 |
| 21 |
Ghasemvand F , Omidinia E , Salehi Z , et al. Relationship between polymorphisms in the proline dehydrogenase gene and schizophrenia risk[J]. Genet Mol Res, 2015, 14 (4): 11681- 11691.
doi: 10.4238/2015.October.2.1 |
| 22 |
Clelland CL , Drouet V , Rilett KC , et al. Evidence that COMT genotype and proline interact on negative-symptom outcomes in schizophrenia and bipolar disorder[J]. Transl Psychiatry, 2016, 6 (9): e891.
doi: 10.1038/tp.2016.157 |
| 23 | Pickar D , Breier A , Kelsoe J . Plasma homovanillic acid as an index of central dopaminergic activity: Studies in schizophrenic patients[J]. Ann N Y Acad Sci, 1988, 537 (1): 339- 346. |
| 24 |
Yao JK , Dougherty GG , Reddy RD , et al. Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia[J]. Mol Psychiatry, 2010, 15 (9): 938- 953.
doi: 10.1038/mp.2009.33 |
| 25 |
Firth J , Stubbs B , Sarris J , et al. The effects of vitamin and mineral supplementation on symptoms of schizophrenia: A systematic review and meta-analysis[J]. Psychol Med, 2017, 47 (9): 1515- 1527.
doi: 10.1017/S0033291717000022 |
| 26 |
Tomioka Y , Numata S , Kinoshita M , et al. Decreased serum pyridoxal levels in schizophrenia: Meta-analysis and Mendelian randomization analysis[J]. J Psychiatry Neurosci, 2018, 43 (3): 194- 200.
doi: 10.1503/jpn.170053 |
| [1] | Ren LONG,Xin MAO,Tianzi GAO,Qian XIE,Hanbo TAN,Ziyin LI,Hongbin HAN,Lan YUAN. Ursolic acid improved demyelination and interstitial fluid drainage disorders in schizophrenia mice [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 487-494. |
| [2] | Hai-hong JIANG,Xiao-fan LI,Jian-liu WANG. Relationship between chronic radiation enteritis of cervical cancer and gut microbiota [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 619-624. |
| [3] | BAI Ning,YANG Ling-fei,AN Li-hua,WANG wen,LI Yun-qian,SHENG Hui,WANG Tong,LI Hua-kan,YUAN Lan. Dynamic visual analysis of neutrophils chemotaxis in peritoneal cavity of schizophrenic model in mice [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 226-230. |
|
||