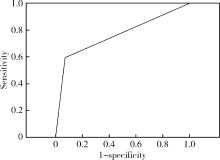
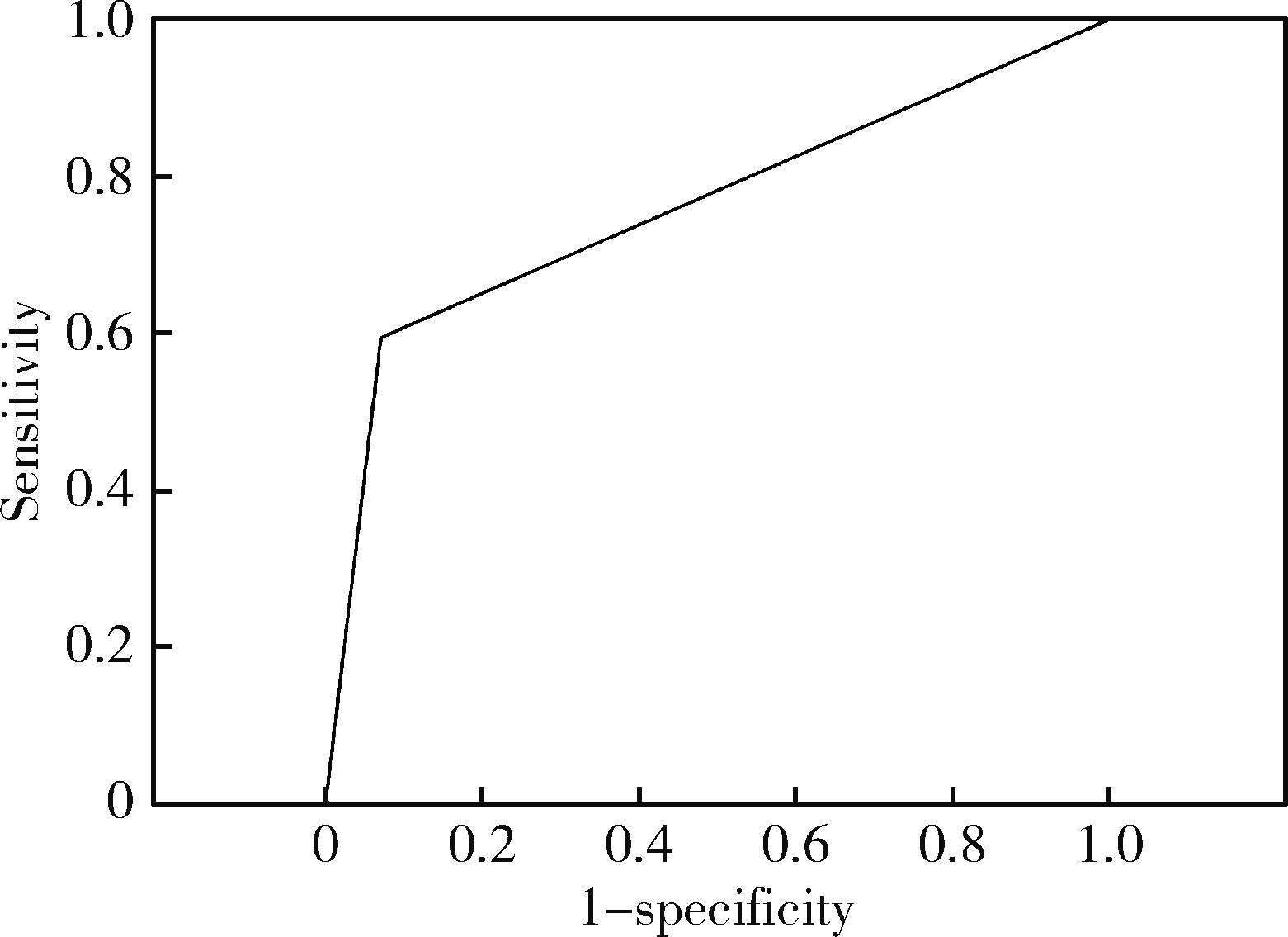
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (6): 1123-1127. doi: 10.19723/j.issn.1671-167X.2022.06.011
Previous Articles Next Articles
Diagnostic performances of salivary gland ultrasonography for Sjögren's syndrome
Yang LIU,Fang CHENG,Yan-ling WANG,Xiang-yan AI,Zhen-hang ZHU,Fu-tao ZHAO*( )
)
- Department of Rheumatology and Immunology, the Ninth People's Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 201999, China
CLC Number:
- R593.2
| 1 |
Shiboski CH , Shiboski SC , Seror R , et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren's syndrome: A consensus and data-driven methodology involving three international patient cohorts[J]. Arthritis Rheumatol, 2017, 69 (1): 35- 45.
doi: 10.1002/art.39859 |
| 2 |
Theander E , Mandl T . Primary Sjögren's syndrome: Diagnostic and prognostic value of salivary gland ultrasonography using a simplified scoring system[J]. Arthritis Care Res (Hoboken), 2014, 66 (7): 1102- 1107.
doi: 10.1002/acr.22264 |
| 3 |
Salaffi F , Carotti M , Iagnocco A , et al. Ultrasonography of salivary glands in primary Sjögren's syndrome: A comparison with contrast sialography and scintigraphy[J]. Rheumatology (Oxford), 2008, 47 (8): 1244- 1249.
doi: 10.1093/rheumatology/ken222 |
| 4 | Lee KA , Lee SH , Kim HR , et al. Diagnostic and predictive eva-luation using salivary glandultrasonography in primary Sjögren's syndrome[J]. Clin Exp Rheumatol, 2018, 112 (3): 165- 172. |
| 5 |
Jousse-Joulin S , Gatineau F , Baldini C , et al. Weight of salivary gland ultrasonography compared toother items of the 2016 ACR/EULAR classification criteria for Primary Sjögren's syndrome[J]. J Intern Med, 2020, 287 (2): 180- 188.
doi: 10.1111/joim.12992 |
| 6 |
van Nimwegen JF , Mossel E , Delli K , et al. Incorporation of salivary gland ultrasonography into the ACR EULAR criteria for primary Sjögren's syndrome[J]. Arthritis Care Res (Hoboken), 2020, 72 (4): 583- 590.
doi: 10.1002/acr.24017 |
| 7 |
Takagi Y , Nakamura H , Sumi M , et al. Combined classification system based on ACR/EULAR and ultrasonographic scores for improving the diagnosis of Sjögren's syndrome[J]. PLoS One, 2018, 13 (4): e0195113.
doi: 10.1371/journal.pone.0195113 |
| 8 |
Jousse-Joulin S , D'Agostino MA , Nicolas C , et al. Video clip assessment of a salivary gland ultrasound scoring system in Sjögren's syndrome using consensual definitions: An OMERACT uhrasound working group reliability exercise[J]. Ann Rheum Dis, 2019, 78 (7): 967- 973.
doi: 10.1136/annrheumdis-2019-215024 |
| 9 |
Zhou M , Song S , Wu S , et al. Diagnostic accuracy of salivary gland ultrasonography with different scoring systems in Sjögren's syndrome: A systematic review and meta-analysis[J]. Sci Rep, 2018, 8 (1): 17128.
doi: 10.1038/s41598-018-35288-5 |
| 10 | Ramsubeik K , Motilal S , Sanchez-Ramos L , et al. Diagnostic accuracy of salivary gland ultrasound in Sjögren's syndrome: A systematic review and meta-analysis[J]. Ther Adv Musculoskelet Dis, 2020, 12, 1- 21. |
| 11 |
Jousse-Joulin S , Milic V , Jonsson MV , et al. Is salivary gland ultrasonography a useful tool in Sjögren's syndrome? A systematic review[J]. Rheumatology (Oxford), 2016, 55 (5): 789- 800.
doi: 10.1093/rheumatology/kev385 |
| 12 | Carotti M , Sala F , Di Carlo M , et al. Diagnostic value of major salivary gland ultrasonography in primary Sjögren's syndrome: The role of grey-scale and colour/power Doppler sonography[J]. Gland Surg, 2019, 8 (Suppl 3): S159- S167. |
| 13 |
Mossel E , Arends S , van Nimwegen JF , et al. Scoring hypoechogenic areas in one parotid and one submandibular gland increases feasibility of ultrasound in primary Sjögren's syndrome[J]. Ann Rheum Dis, 2018, 77 (4): 556- 562.
doi: 10.1136/annrheumdis-2017-211992 |
| 14 |
Izzetti R , Fulvio G , Nisi M , et al. Reliability of OMERACT scoring system in ultra-high frequency ultrasonography of minor salivary glands: Inter-rater agreement study[J]. J Imaging, 2022, 8 (4): 111.
doi: 10.3390/jimaging8040111 |
| 15 |
Al Tabaa O , Gouze H , Hamroun S , et al. Normal salivary gland ultrasonography could rule out the diagnosis of Sjögren's syndrome in anti-SSA-negative patients with sicca syndrome[J]. RMD Open, 2021, 7 (1): e001503.
doi: 10.1136/rmdopen-2020-001503 |
| 16 |
Fana V , Dohn UM , Krabbe S , et al. Application of the OMERACT grey-scale ultrasound scoring system for salivary glands in a single-centre cohort of patients with suspected Sjögren's syndrome[J]. RMD Open, 2021, 7 (2): e001516.
doi: 10.1136/rmdopen-2020-001516 |
| 17 | Robin F, Albert JD, Lescoat A, et al. Diagnostic performances of ultrasound evaluation of major salivary glands according to the 2019 OMERACT US scoring system[J/OL]. Arthritis Care Res (Hoboken), 2021, 5 (2021-05-10)[2022-04-15]. https://onlinelibrary.wiley.com/doi/10.1002/acr.24631. |
| 18 |
Gazeau P , Cornec D , Jousse-Joulin S , et al. Time-course of ultrasound abnormalities of major salivary glands in suspected Sjögren's syndrome[J]. Joint Bone Spine, 2018, 85 (2): 227- 232.
doi: 10.1016/j.jbspin.2017.02.007 |
| 19 | Zandonella-Callegher S , Zabotti A , Giovannini I , et al. Normal-appearing salivary gland ultrasonography identifies a milder phenotype of primary Sjögren's syndrome[J]. Front Med (Lausanne), 2020, 7, 602354. |
| 20 |
Mossel E , Delli K , Nimwegen GF , et al. Ultrasonography of major salivary glands compared with parotid and labial gland biopsy and classification criteria in patients with clinically suspected primary Sjögren's syndrome[J]. Ann Rheum Dis, 2017, 76 (11): 1883- 1889.
doi: 10.1136/annrheumdis-2017-211250 |
| 21 |
Kim JW , Lee H , Park SH , et al. Salivary gland ultrasonography findings are associated with clinical, histological, and serologic features of Sjögren's syndrome[J]. Scand J Rheumatol, 2018, 47 (4): 303- 310.
doi: 10.1080/03009742.2017.1374451 |
| 22 |
Jousse-Joulin S , Devauchelle-Pensec V , Cornec D , et al. Brief report: Ultrasonographic assessment of salivary gland response to rituximab in primary Sjögren's syndrome[J]. Arthritis Rheumatol, 2015, 67 (6): 1623- 1628.
doi: 10.1002/art.39088 |
| 23 |
Lorenzon M , Spina E , Di Franco FT , et al. Salivary gland ultrasound in primary Sjögren's syndrome: Current and future perspectives[J]. Open Access Rheumatol, 2022, 14, 147- 160.
doi: 10.2147/OARRR.S284763 |
| [1] | Yushu YANG, Xuan QI, Meng DING, Wei WANG, Huifang GUO, Lixia GAO. Diagnostic values of anti-salivary gland protein-1 antibody combined with anti-parotid secretory protein antibody for Sjögren's syndrome [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 845-852. |
| [2] | Shu-heng ZHAI,Pan-pan HU,Xiao-guang LIU. Intraoperative ultrasound assisted circumferential decompression for multilevel ossification of the posterior longitudinal ligament in thoracic vertebrae [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 1021-1027. |
| [3] | YU Guang-yan,LIU Deng-gao,LI Wei,HONG Xia,ZHANG Yan-yan,ZHU Wen-xuan,ZHANG Ke-fu,LI Xiao,LI Zhan-guo,LIU Yan-ying,CHEN Yan,GAO Yan,SU Jia-zeng. Studies on newly recognized chronic sialadenitis [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 13-17. |
| [4] | DENG Xue-rong,SUN Xiao-ying,ZHANG Zhuo-li. Agreement between ultrasound-detected inflammation and clinical signs in ankles and feet joints in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1037-1042. |
| [5] | SONG Zhi-bo,GENG Yan,DENG Xue-rong,ZHANG Xiao-hui,ZHANG Zhuo-li. Benefit of ultrasound in the phenotype recognition of psoriatic arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1061-1066. |
| [6] | PENG Zhe,DING Ya-min,PEI Lin,YAO Hai-hong,ZHANG Xue-wu,TANG Su-mei. Clinical characteristics of crystal deposits in joints and tendons in patients with gout [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1067-1071. |
| [7] | ZHU Yi-ying,MIN Sai-nan,YU Guang-yan. Effect of topical injection of cyclosporine A on saliva secretion and inflammation in the submandibular gland of non-obese diabetic mice [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 750-757. |
| [8] | Huan-bin YU,Wen-jie WU,Xiao-ming LV,Yan SHI,Lei ZHENG,Jian-guo ZHANG. 125I seed brachytherapy for recurrent salivary gland carcinoma after external radiotherapy [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 919-923. |
| [9] | Ning KANG,Yi-hang JIANG,Yu-guang JIANG,Li-yang WU,Ji-qing ZHANG,Yi-nong NIU,Jun-hui ZHANG. Endoscopic combined ultrasound-guided access vs. ultrasound-guided access in endoscopic combined intrarenal surgery [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 692-696. |
| [10] | Hao WANG,Shu-kun JIANG,Ran PENG,Yi HUANG,Ming-qing WANG,Jun-jie WANG,Cheng LIU,Fan ZHANG,Lu-lin MA. Individual control of urine volume to improve stability of bladder volume in radiotherapy of urinary tumor [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 688-691. |
| [11] | Ye ZHANG,Ni ZHANG,Xiao-xiao LIU,Chuan-xiang ZHOU. Cervical lymph node metastasis in adenoid cystic carcinoma of the salivary glands: A clinicopathologic study [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 30-34. |
| [12] | Yan GENG,Bo-rui LI,Zhuo-li ZHANG. Musculoskeletal ultrasound findings of symptomatic joints in patients with systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 163-168. |
| [13] | Li-wei LI,Zhuo LIU,Guo-liang WANG,Hua ZHANG,Wen CHEN,Jing MA,Li ZHANG,Wei HE,Lu-lin MA,Shu-min WANG. Comparison of various imaging in the diagnosis of renal cell carcinoma with inferior vena cava tumor thrombus combined with bland thrombus [J]. Journal of Peking University(Health Sciences), 2019, 51(4): 678-683. |
| [14] | Cui-ping ZHANG,Pei-pei LIU,Qiang FU,Guan-ying GAO,Li-gang CUI,Yan XU,Jian-quan WANG. Application of ultrasound-guided hip joint drug injection in the postoperative rehabilitation of arthroscopie repair of acetabular labral tears [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 265-267. |
| [15] | Guang-ya YU,Xia HONG,Wei LI,Yan-yan ZHANG,Yan GAO,Yan CHEN,Zu-yan ZHANG,Xiao-yan XIE,Zhan-guo LI,Yan-ying LIU,Jia-zeng SU,Wen-xuan ZHU,Zhi-peng SUN. Clinicopathological characteristics and diagnosis of IgG4-related sialadenitis [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 1-3. |
|
||