Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (1): 120-130. doi: 10.19723/j.issn.1671-167X.2024.01.019
Previous Articles Next Articles
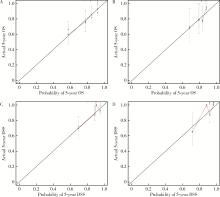
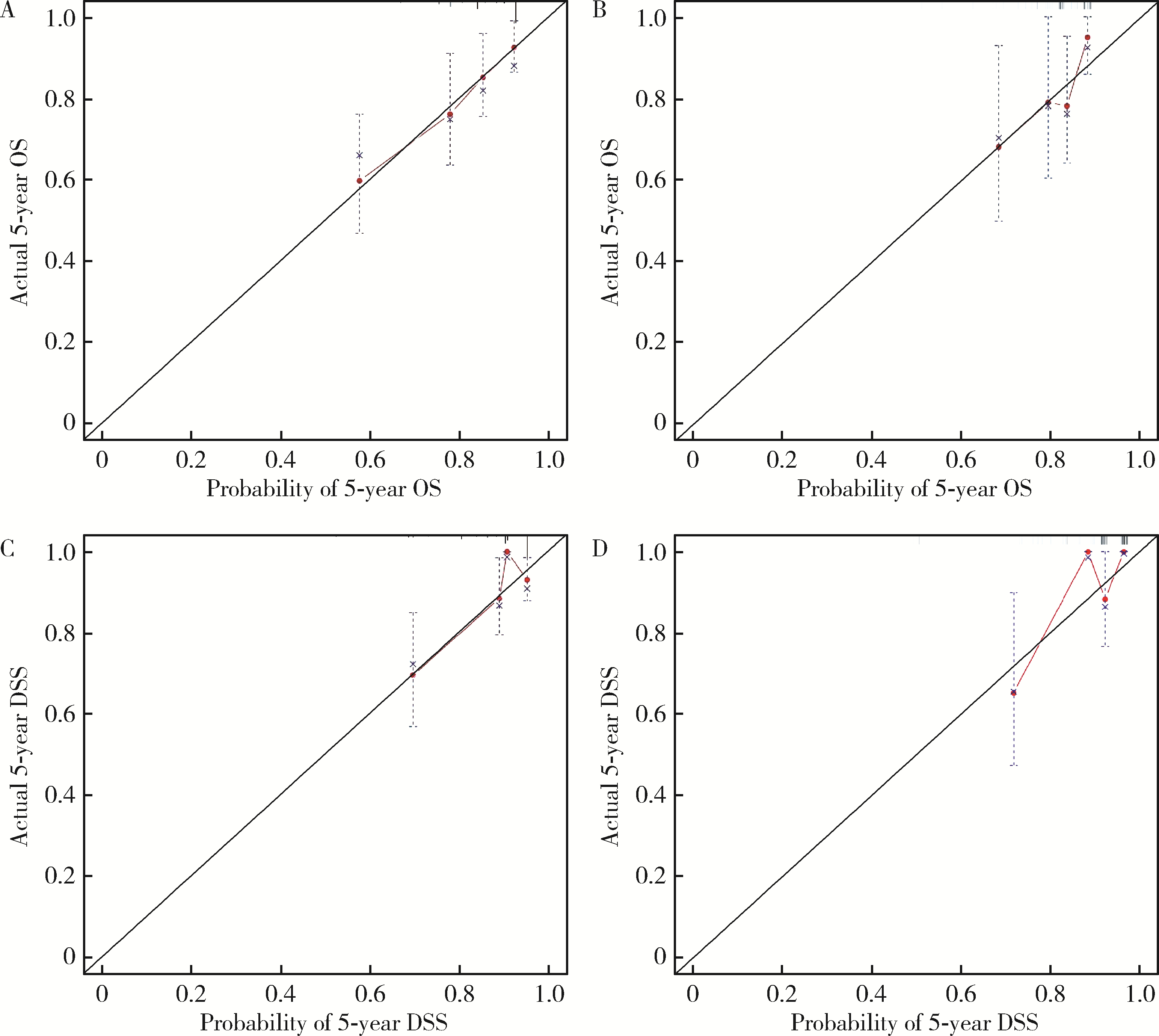
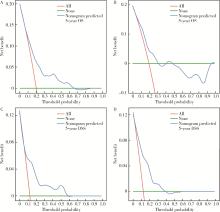
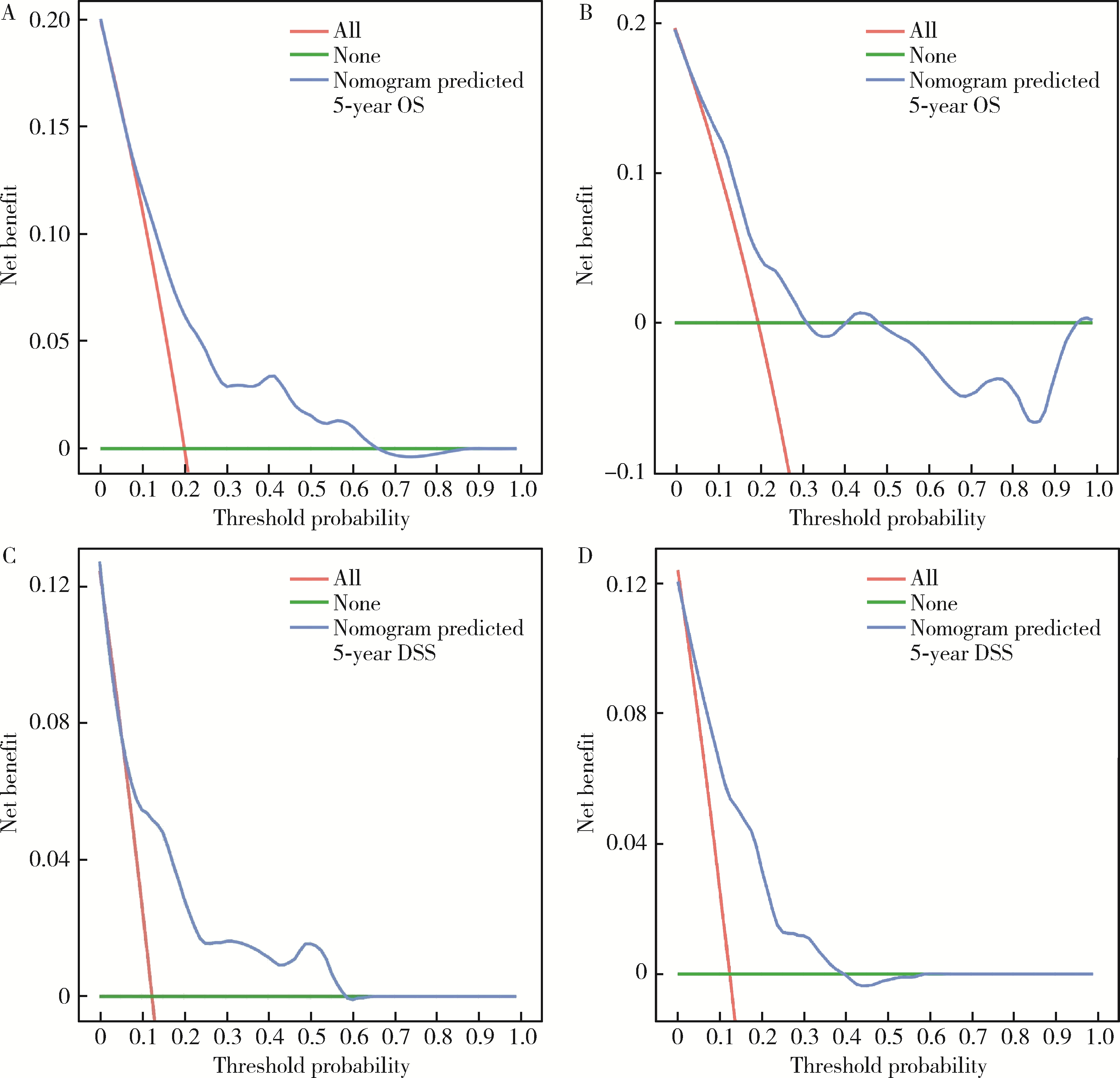
Establishment and verification of a prognostic nomogram for survival of tongue squamous cell carcinoma patients who underwent cervical dissection
Junqi SU1,Xiaoying WANG2,Zhiqiang SUN1,*( )
)
- 1. Department of Clinical Laboratory, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices, Beijing 100081, China
2. Department of Medical Record, Peking University School and Hospital of Stomatology & National Center for Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices, Beijing 100081, China
CLC Number:
- R739.8
| 1 |
Panarese I , Aquino G , Ronchi A , et al. Oral and oropharyngeal squamous cell carcinoma: Prognostic and predictive parameters in the etiopathogenetic route[J]. Expert Rev Anticancer Ther, 2019, 19 (2): 105- 119.
doi: 10.1080/14737140.2019.1561288 |
| 2 |
Faisal M , Abu Bakar M , Sarwar A , et al. Depth of invasion (DOI) as a predictor of cervical nodal metastasis and local recurrence in early stage squamous cell carcinoma of oral tongue (ESSCOT)[J]. PLoS One, 2018, 13 (8): e0202632.
doi: 10.1371/journal.pone.0202632 |
| 3 |
Ibrahim SA , Ahmed ANA , Elsersy HA , et al. Elective neck dissection in T1/T2 oral squamous cell carcinoma with N0 neck: Essential or not? A systematic review and meta-analysis[J]. Eur Arch Otorhinolaryngol, 2020, 277 (6): 1741- 1752.
doi: 10.1007/s00405-020-05866-3 |
| 4 |
Lu Z , Yan W , Liang J , et al. Nomogram based on systemic immune-inflammation index to predict survival of tongue cancer patients who underwent cervical dissection[J]. Front Oncol, 2020, 10, 341.
doi: 10.3389/fonc.2020.00341 |
| 5 |
Jia B , Dao J , Han J , et al. LINC00958 promotes the proliferation of TSCC via miR-211-5p/CENPK axis and activating the JAK/STAT3 signaling pathway[J]. Cancer Cell Int, 2021, 21 (1): 147.
doi: 10.1186/s12935-021-01808-z |
| 6 |
Almangush A , Pirinen M , Youssef O , et al. Risk stratification in oral squamous cell carcinoma using staging of the eighth American Joint Committee on Cancer: Systematic review and meta-analysis[J]. Head Neck, 2020, 42 (10): 3002- 3017.
doi: 10.1002/hed.26344 |
| 7 |
Wei PY , Li WY , Tai SK . Discrete perineural invasion focus number in quantification for T1-T2 oral squamous cell carcinoma[J]. Otolaryngol Head Neck Surg, 2019, 160 (4): 635- 641.
doi: 10.1177/0194599818808510 |
| 8 |
Shi J , Bao X , Liu Z , et al. Serum miR-626 and miR-5100 are promising prognosis predictors for oral squamous cell carcinoma[J]. Theranostics, 2019, 9 (4): 920- 931.
doi: 10.7150/thno.30339 |
| 9 |
Romani C , Salviato E , Paderno A , et al. Genome-wide study of salivary miRNAs identifies miR-423-5p as promising diagnostic and prognostic biomarker in oral squamous cell carcinoma[J]. Theranostics, 2021, 11 (6): 2987- 2999.
doi: 10.7150/thno.45157 |
| 10 |
Zhang J , Hu Z , Horta CA , et al. Regulation of epithelial-mesenchymal transition by tumor microenvironmental signals and its implication in cancer therapeutics[J]. Semin Cancer Biol, 2023, 88, 46- 66.
doi: 10.1016/j.semcancer.2022.12.002 |
| 11 |
Mariani P , Russo D , Maisto M , et al. Pre-treatment neutrophil-to-lymphocyte ratio is an independent prognostic factor in head and neck squamous cell carcinoma: Meta-analysis and trial sequential analysis[J]. J Oral Pathol Med, 2022, 51 (1): 39- 51.
doi: 10.1111/jop.13264 |
| 12 |
Kumarasamy C , Tiwary V , Sunil K , et al. Prognostic utility of platelet-lymphocyte ratio, neutrophil-lymphocyte ratio and monocyte-lymphocyte ratio in head and neck cancers: A detailed PRISMA compliant systematic review and meta-analysis[J]. Cancers (Basel), 2021, 13 (16): 4166.
doi: 10.3390/cancers13164166 |
| 13 |
Xu X , Jing J . Inflammation-related parameter serve as prognostic biomarker in esophageal squamous cell carcinoma[J]. Front Oncol, 2022, 12, 900305.
doi: 10.3389/fonc.2022.900305 |
| 14 |
Wang Y , Lyu J , Jia H , et al. Clinical utility of the systemic immune-inflammation index for predicting survival in esophageal squamous cell carcinoma after radical radiotherapy[J]. Future Oncol, 2021, 17 (20): 2647- 2657.
doi: 10.2217/fon-2021-0304 |
| 15 |
Wang D , Hu X , Xiao L , et al. Prognostic nutritional index and systemic immune-inflammation index predict the prognosis of patients with HCC[J]. J Gastrointest Surg, 2021, 25 (2): 421- 427.
doi: 10.1007/s11605-019-04492-7 |
| 16 |
Murthy P , Zenati MS , Al Abbas AI , et al. Prognostic value of the systemic immune-inflammation index (SII) after neoadjuvant therapy for patients with resected pancreatic cancer[J]. Ann Surg Oncol, 2020, 27 (3): 898- 906.
doi: 10.1245/s10434-019-08094-0 |
| 17 |
Chang Y , An H , Xu L , et al. , Systemic inflammation score predicts postoperative prognosis of patients with clear-cell renal cell carcinoma[J]. Br J Cancer, 2015, 113 (4): 626- 633.
doi: 10.1038/bjc.2015.241 |
| 18 |
Kim MG , Choi YS , Youn SM , et al. Treatment outcomes and prognostic factors in oral tongue cancer: A 20-year retrospective study at the National Cancer Center, South Korea[J]. J Korean Assoc Oral Maxillofac Surg, 2022, 48 (4): 192- 200.
doi: 10.5125/jkaoms.2022.48.4.192 |
| 19 |
Wolfer S , Elstner S , Schultze-Mosgau S . Degree of keratinization is an independent prognostic factor in oral squamous cell carcinoma[J]. J Oral Maxillofac Surg, 2018, 76 (2): 444- 454.
doi: 10.1016/j.joms.2017.06.034 |
| 20 |
Ravasco P , Monteiro-Grillo I , Vidal PM , et al. Nutritional deterioration in cancer: The role of disease and diet[J]. Clin Oncol (R Coll Radiol), 2003, 15 (8): 443- 450.
doi: 10.1016/S0936-6555(03)00155-9 |
| 21 |
Jiang Y , Xu D , Song H , et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: A systematic review and meta-analysis[J]. BMJ Open, 2021, 11 (9): e048324.
doi: 10.1136/bmjopen-2020-048324 |
| 22 |
Kono T , Sakamoto K , Shinden S , et al. Pre-therapeutic nutri-tional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy[J]. Clin Nutr, 2017, 36 (6): 1681- 1685.
doi: 10.1016/j.clnu.2016.10.021 |
| 23 |
Yoshimura T , Suzuki H , Takayama H , et al. Prognostic value of inflammatory biomarkers in aged patients with oral squamous cell carcinoma[J]. Front Pharmacol, 2022, 13, 996757.
doi: 10.3389/fphar.2022.996757 |
| 24 |
Kubota K , Ito R , Narita N , et al. Utility of prognostic nutritional index and systemic immune-inflammation index in oral cancer treatment[J]. BMC Cancer, 2022, 22 (1): 368.
doi: 10.1186/s12885-022-09439-x |
| [1] | Zhicun LI, Tianyu WU, Lei LIANG, Yu FAN, Yisen MENG, Qian ZHANG. Risk factors analysis and nomogram model construction of postoperative pathological upgrade of prostate cancer patients with single core positive biopsy [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 896-901. |
| [2] | Yuanmei LIU, Yicheng FU, Jingxin HAO, Fuchun ZHANG, Huilin LIU. Construction and validation of a nomogram for predicting in-hospital postoperative heart failure in elderly patients with hip fracture [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 874-883. |
| [3] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [4] | Hai MAO,Fan ZHANG,Zhan-yi ZHANG,Ye YAN,Yi-chang HAO,Yi HUANG,Lu-lin MA,Hong-ling CHU,Shu-dong ZHANG. Predictive model of early urinary continence recovery based on prostate gland MRI parameters after laparoscopic radical prostatectomy [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 818-824. |
| [5] | Mei-ni ZUO,Yi-qing DU,Lu-ping YU,Xiang DAI,Tao XU. Correlation between metabolic syndrome and prognosis of patients with clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 636-643. |
| [6] | DING Ting-ting,ZENG Chu-xiong,HU Li-na,YU Ming-hua. Establishment of a prediction model for colorectal cancer immune cell infiltration based on the cancer genome atlas (TCGA) database [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 203-208. |
| [7] | Wen-peng WANG,Jie-fu WANG,Jun HU,Jun-feng WANG,Jia LIU,Da-lu KONG,Jian LI. Clinicopathological features and prognosis of colorectal stromal tumor [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 353-361. |
| [8] | LIU Yu-qing, LU Jian, HAO Yi-chang, XIAO Chun-lei, MA Lu-lin. Predicting model based on risk factors for urosepsis after percutaneous nephrolithotomy [J]. Journal of Peking University(Health Sciences), 2018, 50(3): 507-513. |
|
||