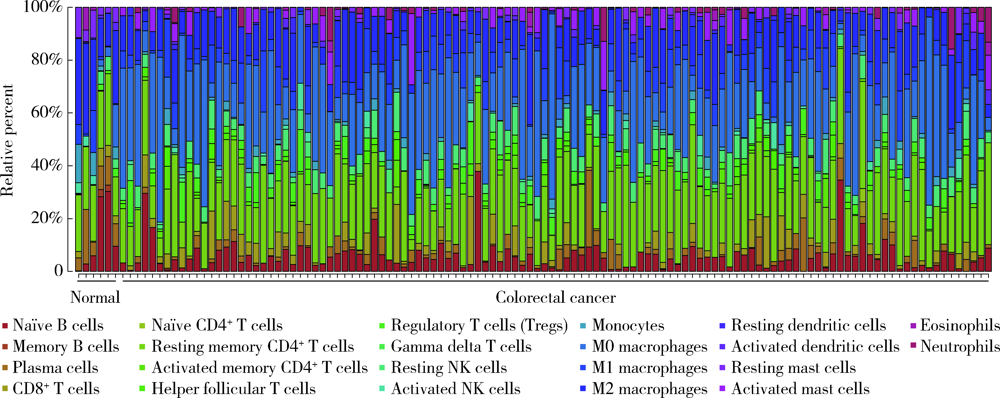
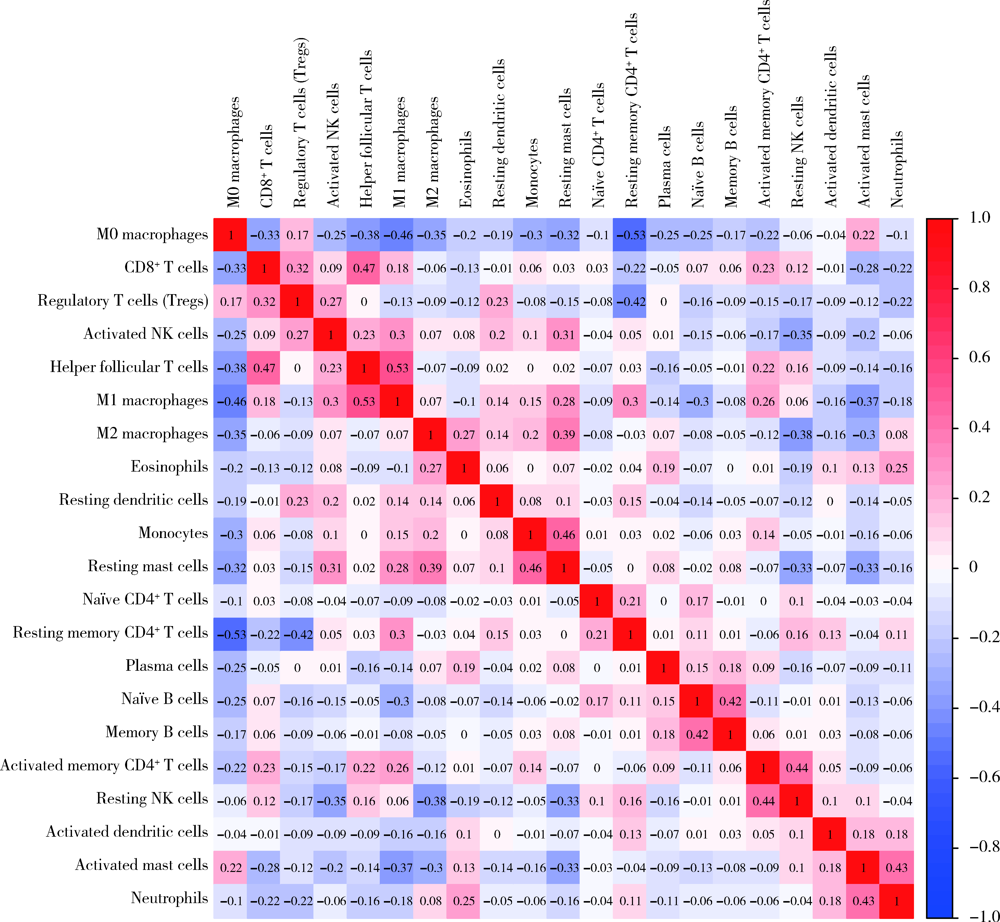
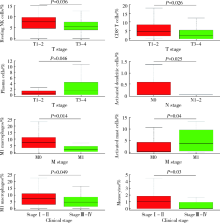
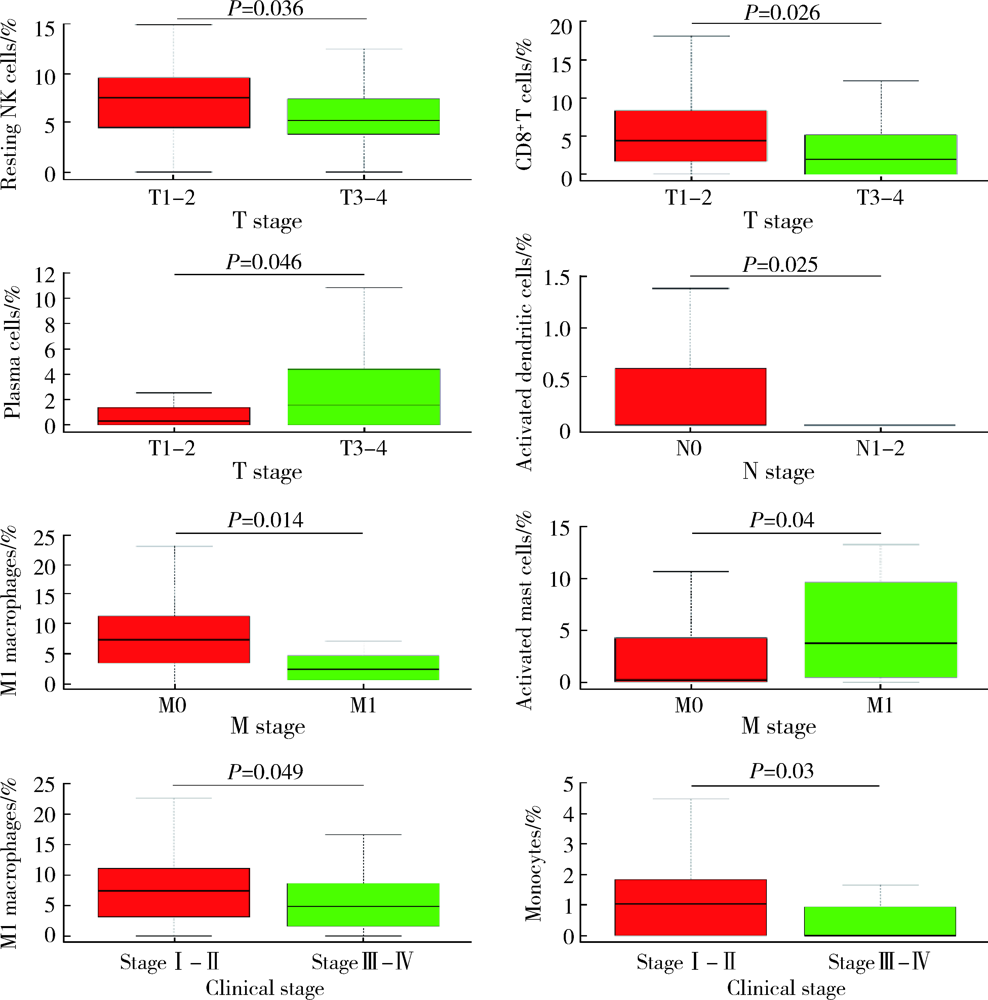
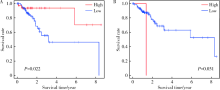
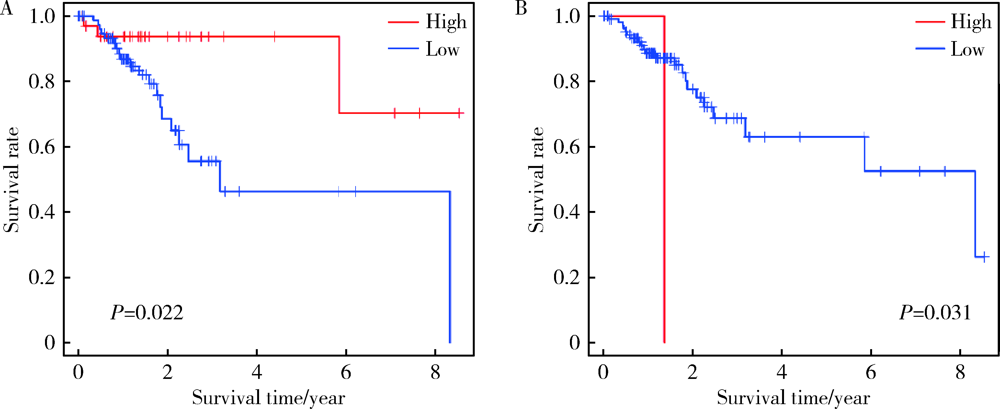
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (2): 203-208. doi: 10.19723/j.issn.1671-167X.2022.02.001
Establishment of a prediction model for colorectal cancer immune cell infiltration based on the cancer genome atlas (TCGA) database
DING Ting-ting,ZENG Chu-xiong,HU Li-na,YU Ming-hua( )
)
- Department of Oncology, Shanghai Pudong Hospital, Pudong Hospital Affiliated to Fudan University, Shanghai 201399, China
CLC Number:
- R730.51
| [1] |
Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017[J]. CA Cancer J Clin, 2017, 67(3):177-193.
doi: 10.3322/caac.21395 |
| [2] | Shibutani M, Maeda K, Nagahara H, et al. Tumor-infiltrating lymphocytes predict the chemotherapeutic outcomes in patients with stage Ⅳ colorectal cancer[J]. In Vivo, 2018, 32(1):151-158. |
| [3] |
Guinney J, Dienstmann R, Wang X, et al. The consensus mole-cular subtypes of colorectal cancer[J]. Nat Med, 2015, 21(11):1350-1356.
doi: 10.1038/nm.3967 pmid: 26457759 |
| [4] |
Church J. Molecular genetics of colorectal cancer[J]. Sem Colon Rectal Surg, 2016, 27(4):172-175.
doi: 10.1053/j.scrs.2016.04.013 |
| [5] |
Bremnes RM, Al-Shibli K, Donnem T, et al. The role of tumor-infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: Emphasis on non-small cell lung cancer[J]. J Thorac Oncol, 2011, 6(4):824-833.
doi: 10.1097/JTO.0b013e3182037b76 pmid: 21173711 |
| [6] |
Mao X, Xu J, Wang W, et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives[J]. Mol Cancer, 2021, 20(1):131-142.
doi: 10.1186/s12943-021-01428-1 |
| [7] |
Baxevanis CN, Papamichail M, Perez SA. Immune classification of colorectal cancer patients: Impressive but how complete?[J]. Expert Opin Biol Ther, 2013, 13(4):517-526.
doi: 10.1517/14712598.2013.751971 |
| [8] |
Grizzi F, Basso G, Borroni EM, et al. Evolving notions on immune response in colorectal cancer and their implications for biomarker developmentc[J]. Inflamm Res, 2018, 67(5):375-389.
doi: 10.1007/s00011-017-1128-1 pmid: 29322204 |
| [9] |
Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles[J]. Nat Methods, 2015, 12(5):453-457.
doi: 10.1038/nmeth.3337 pmid: 25822800 |
| [10] |
Liu X, Wu S, Yang Y, et al. The prognostic landscape of tumor-infiltrating immune cell and immunomodulators in lung cancer[J]. Biomed Pharmacother, 2017, 95:55-61.
doi: 10.1016/j.biopha.2017.08.003 |
| [11] |
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis[J]. Nat Med, 2013, 19(11):1423-1437.
doi: 10.1038/nm.3394 |
| [12] |
Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer[J]. Trends Cell Biol, 2015, 25(4):198-213.
doi: 10.1016/j.tcb.2014.11.006 |
| [13] |
Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer[J]. Clin Cancer Res, 2014, 20(7):1891-1899.
doi: 10.1158/1078-0432.CCR-13-2830 |
| [14] |
Huh JW, Lee JH, Kim HR. Prognostic significance of tumorinfiltrating lymphocytes for patients with colorectal cancer[J]. Arch Surg, 2012, 147(4):366-372.
doi: 10.1001/archsurg.2012.35 |
| [15] |
Karpinski P, Rossowska J, Sasiadek MM. Immunological landscape of consensus clusters in colorectal cancer[J]. Oncotarget, 2017, 8(62):105299-105311.
doi: 10.18632/oncotarget.v8i62 |
| [16] |
Mirjolet C, Charon-Barra C, Ladoire S, et al. Tumor lymphocyte immune response to preoperative radiotherapy in locally advanced rectal cancer: The LYMPHOREC study[J]. Oncoimmunology, 2018, 7(3):e1396402.
doi: 10.1080/2162402X.2017.1396402 |
| [17] |
Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome[J]. Science, 2006, 313(5759):1960-1964.
doi: 10.1126/science.1129139 |
| [18] |
Klintrup K, Makinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer[J]. Eur J Cancer, 2005, 41(17):2645-2654.
doi: 10.1016/j.ejca.2005.07.017 pmid: 16239109 |
| [19] |
Li T, Fan J, Wang B, et al. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells[J]. Cancer Res, 2017, 77(21):e108-e110.
doi: 10.1158/0008-5472.CAN-17-0307 |
| [20] |
Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus immunoscore for the classification of colon cancer: A prognostic and accuracy study[J]. Lancet, 2018, 391(10135):2128-2139.
doi: 10.1016/S0140-6736(18)30789-X |
| [21] |
Steinman RM. Decisions about dendritic cells: Past, present, and future[J/OL]. Annu Rev Immunol, 2012, 30:1-22. doi: 10.1146/annurevimmunol-100311-102839.
doi: 10.1146/annurevimmunol-100311-102839 |
| [22] |
Shimizu K, Kotera Y, Aruga A, et al. Postoperative dendritic cell vaccine plus activated T-cell transfer improves the survival of patients with invasive hepatocellular carcinoma[J]. Hum Vaccin Immunother, 2014, 10(4):970-976.
doi: 10.4161/hv.27678 |
| [23] | Pagès F, Kirilovsky A, Mlecnik B, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer[J]. J Clin Oncol, 2009, 27(35):5944-5951. |
| [24] |
Gannon PO, Baumgaertner P, Huber A, et al. Rapid and continued T-cell differentiation into long-term effector and memory stem cells in vaccinated melanoma patients[J]. Clin Cancer Res, 2017, 23(13):3285-3296.
doi: 10.1158/1078-0432.CCR-16-1708 |
| [1] | Yuanmei LIU, Yicheng FU, Jingxin HAO, Fuchun ZHANG, Huilin LIU. Construction and validation of a nomogram for predicting in-hospital postoperative heart failure in elderly patients with hip fracture [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 874-883. |
| [2] | Zhicun LI, Tianyu WU, Lei LIANG, Yu FAN, Yisen MENG, Qian ZHANG. Risk factors analysis and nomogram model construction of postoperative pathological upgrade of prostate cancer patients with single core positive biopsy [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 896-901. |
| [3] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [4] | Xiaodong CHAI,Ziwen SUN,Haishuang LI,Liangyi ZHU,Xiaodan LIU,Yantao LIU,Fei PEI,Qing CHANG. Clinicopathological characteristics of the CD8+ T lymphocytes infiltration and its mechanism in distinct molecular subtype of medulloblastoma [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 512-518. |
| [5] | Junqi SU,Xiaoying WANG,Zhiqiang SUN. Establishment and verification of a prognostic nomogram for survival of tongue squamous cell carcinoma patients who underwent cervical dissection [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 120-130. |
| [6] | Hai MAO,Fan ZHANG,Zhan-yi ZHANG,Ye YAN,Yi-chang HAO,Yi HUANG,Lu-lin MA,Hong-ling CHU,Shu-dong ZHANG. Predictive model of early urinary continence recovery based on prostate gland MRI parameters after laparoscopic radical prostatectomy [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 818-824. |
| [7] | Li LIANG,Xin LI,Lin NONG,Ying DONG,Ji-xin ZHANG,Dong LI,Ting LI. Analysis of microsatellite instability in endometrial cancer: The significance of minimal microsatellite shift [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 254-261. |
| [8] | Xiao-jing CHENG,Dong JIANG,Lian-hai ZHANG,Jiang-hua WANG,Ya-zhen LI,Jia-hui ZHAI,Bao-qi YAN,Lu-lu ZHANG,Xing-wang XIE,Zi-yu LI,Jia-fu JI. Preclinical study of T cell receptor specifically reactive with KRAS G12V mutation in the treatment of malignant tumors [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 884-895. |
| [9] | TIAN Jia-yi,ZHANG Xia,CHENG Gong,LIU Qing-hong,WANG Shi-yang,HE Jing. Serum interleukin-2 receptor α as a clinical biomarker in patients with systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1083-1087. |
| [10] | MA Xiang-bo,ZHANG Xue-wu,JIA Ru-lin,GAO Ying,LIU Hong-jiang,LIU Yu-fang,LI Ying-ni. Application of lymphocytes test in peripheral blood of patients with systemic sclerosis during the treatment [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 721-727. |
| [11] | Wen-peng WANG,Jie-fu WANG,Jun HU,Jun-feng WANG,Jia LIU,Da-lu KONG,Jian LI. Clinicopathological features and prognosis of colorectal stromal tumor [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 353-361. |
| [12] | Xu-chu ZHANG,Jian-hua ZHANG,Rong-fu WANG,Yan FAN,Zhan-li FU,Ping YAN,Guang-yu ZHAO,Yan-xia BAI. Diagnostic value of 18F-FDG PET/CT and tumor markers (CEA, CA19-9, CA24-2) in recurrence and metastasis of postoperative colorectal moderately differentiated adenocarcinoma [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1071-1077. |
| [13] | Yu-bing XIAO,Mu-yao GUO,Xiao-xia ZUO. Immunometabolism and systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1120-1124. |
| [14] | ZHOU Jian-hua, WANG Di, WANG Huan-rui, HOU Xiao-li,YU Wei-dong, XU Ke-xin, HU Hao. Cytotoxic effects of γδT cells on bladder cancer cells and expression of MICA/B in bladder cancer [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 595-601. |
| [15] | LIU Yu-qing, LU Jian, HAO Yi-chang, XIAO Chun-lei, MA Lu-lin. Predicting model based on risk factors for urosepsis after percutaneous nephrolithotomy [J]. Journal of Peking University(Health Sciences), 2018, 50(3): 507-513. |
|
||