Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (6): 1009-1016. doi: 10.19723/j.issn.1671-167X.2024.06.010
Previous Articles Next Articles
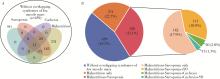
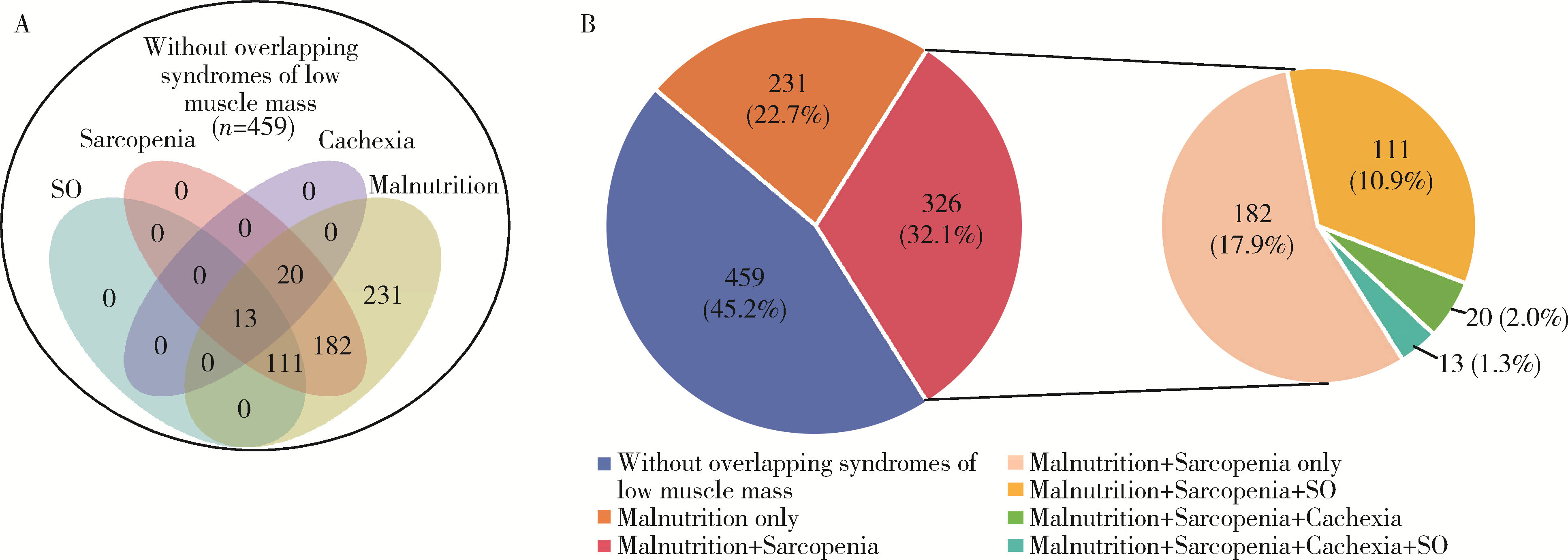
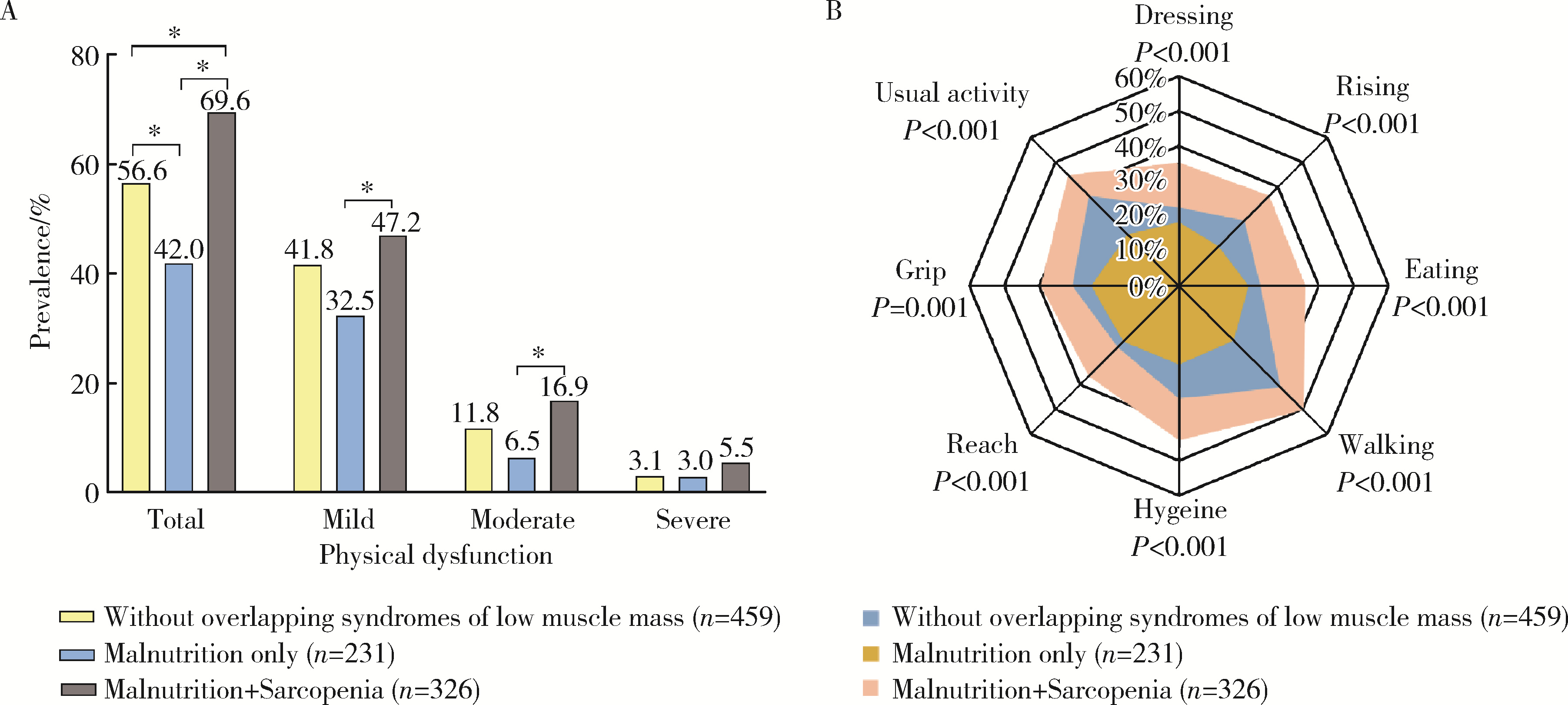
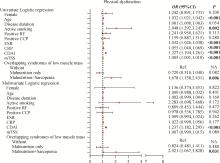
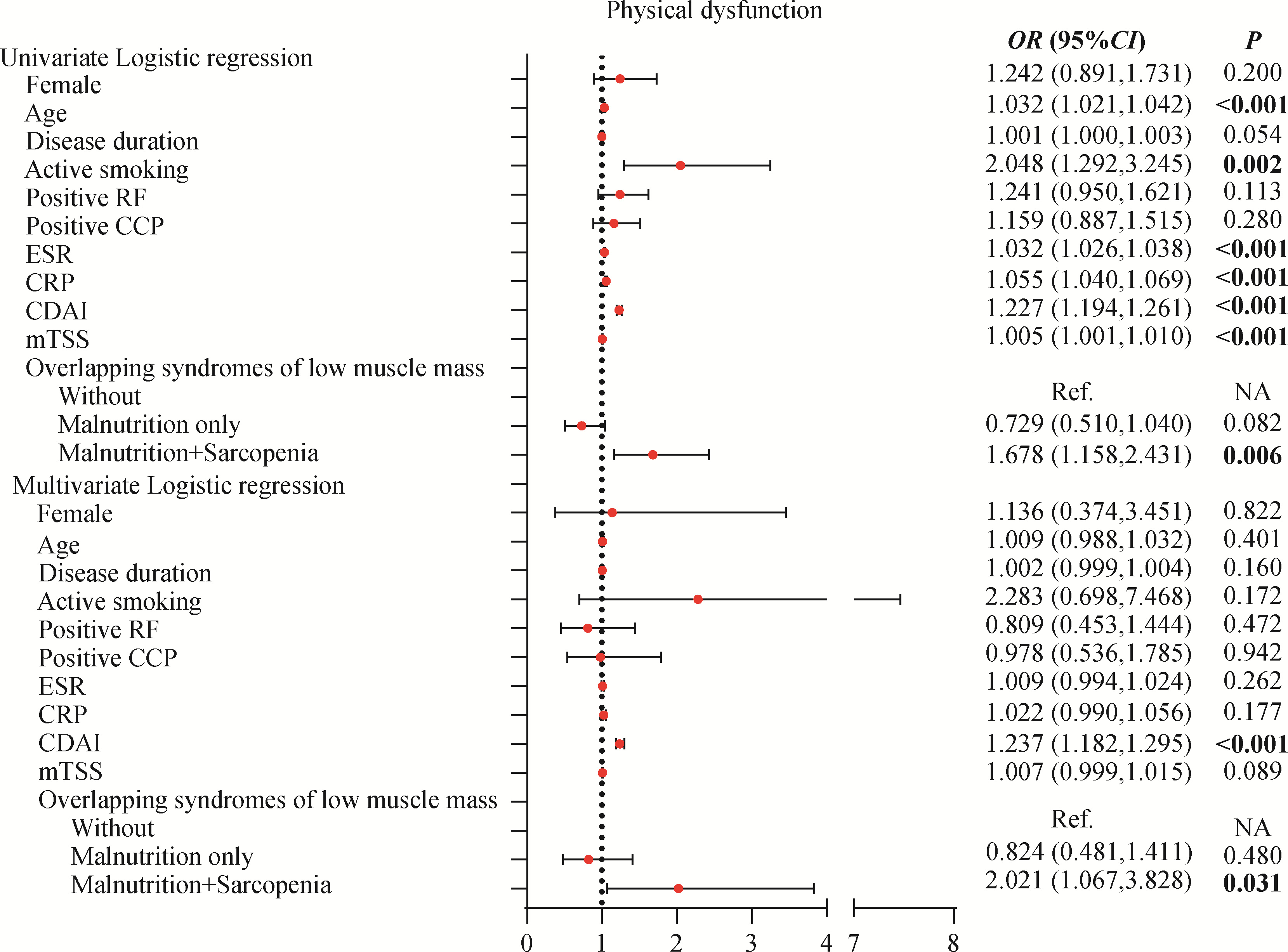
Clinical characteristics of overlapping syndromes of low muscle mass in patients with rheumatoid arthritis and their impact on physical function
Peiwen JIA, Ying YANG, Yaowei ZOU, Zhiming OUYANG, Jianzi LIN, Jianda MA, Kuimin YANG, Lie DAI*( )
)
- Department of Rheumatology and Immunology, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510120, China
CLC Number:
- R593.22
| 1 | 耿研, 谢希, 王昱, 等. 类风湿关节炎诊疗规范[J]. 中华内科杂志, 2022, 61 (1): 51- 59. |
| 2 | 周云杉, 王秀茹, 安媛, 等. 全国多中心类风湿关节炎患者残疾及功能受限情况的调查[J]. 中华风湿病学杂志, 2013, 17 (8): 526- 532. |
| 3 | 邹耀威, 连舒燕, 陈楚涛, 等. 类风湿关节炎患者功能受限特征及相关因素分析[J]. 中华内科杂志, 2022, 61 (2): 193- 199. |
| 4 | Smolen JS , Breedveld FC , Burmester GR , et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force[J]. Ann Rheum Dis, 2015, 75 (1): 3- 15. |
| 5 |
Bennett JL , Pratt AG , Dodds R , et al. Rheumatoid sarcopenia: Loss of skeletal muscle strength and mass in rheumatoid arthritis[J]. Nat Rev Rheumatol, 2023, 19 (4): 239- 251.
doi: 10.1038/s41584-023-00921-9 |
| 6 | Elkan AC , Engvall IL , Tengstrand B , et al. Malnutrition in women with rheumatoid arthritis is not revealed by clinical anthropometrical measurements or nutritional evaluation tools[J]. Eur J Clin Nutr, 2007, 62 (10): 1239- 1247. |
| 7 |
Tański W , Wójciga J , Jankowska-Polańska B . Association between malnutrition and quality of life in elderly patients with rheumatoid arthritis[J]. Nutrients, 2021, 13 (4): 1259.
doi: 10.3390/nu13041259 |
| 8 |
Baker JF , George M , Baker DG , et al. Associations between body mass, radiographic joint damage, adipokines and risk factors for bone loss in rheumatoid arthritis[J]. Rheumatology, 2011, 50 (11): 2100- 2107.
doi: 10.1093/rheumatology/ker294 |
| 9 |
Cederholm T , Jensen GL , Correia MITD , et al. GLIM criteria for the diagnosis of malnutrition: A consensus report from the global clinical nutrition community[J]. Clin Nutr, 2019, 38 (1): 1- 9.
doi: 10.1016/j.clnu.2018.08.002 |
| 10 |
Chew STH , Tey SL , Yalawar M , et al. Prevalence and associated factors of sarcopenia in community-dwelling older adults at risk of malnutrition[J]. BMC Geriatr, 2022, 22 (1): 997.
doi: 10.1186/s12877-022-03704-1 |
| 11 |
Ngeuleu A , Allali F , Medrare L , et al. Sarcopenia in rheumatoid arthritis: Prevalence, influence of disease activity and associated factors[J]. Rheumatol Int, 2017, 37 (6): 1015- 1020.
doi: 10.1007/s00296-017-3665-x |
| 12 |
Giles JT , Ling SM , Ferrucci L , et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: Association with disease characteristics and pharmacotherapies[J]. Arthritis Rheum, 2008, 59 (6): 807- 815.
doi: 10.1002/art.23719 |
| 13 |
Torii M , Hashimoto M , Hanai A , et al. Prevalence and factors associated with sarcopenia in patients with rheumatoid arthritis[J]. Mod Rheumatol, 2019, 29 (4): 589- 595.
doi: 10.1080/14397595.2018.1510565 |
| 14 |
Santo RCE , Fernandes KZ , Lora PS , et al. Prevalence of rheumatoid cachexia in rheumatoid arthritis: A systematic review and meta-analysis[J]. J Cachexia Sarcopenia Muscle, 2018, 9 (5): 816- 825.
doi: 10.1002/jcsm.12320 |
| 15 |
Arnett FC , Edworthy SM , Bloch DA , et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis[J]. Arthritis Rheum, 1988, 31 (3): 315- 324.
doi: 10.1002/art.1780310302 |
| 16 |
Aletaha D , Neogi T , Silman AJ , et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative[J]. Ann Rheum Dis, 2010, 69 (9): 1580- 1588.
doi: 10.1136/ard.2010.138461 |
| 17 |
Lin JZ , Liang JJ , Ma JD , et al. Myopenia is associated with joint damage in rheumatoid arthritis: A cross-sectional study[J]. J Cachexia Sarcopenia Muscle, 2019, 10 (2): 355- 367.
doi: 10.1002/jcsm.12381 |
| 18 |
Oliveros E , Somers VK , Sochor O , et al. The concept of normal weight obesity[J]. Prog Cardiovasc Dis, 2014, 56 (4): 426- 433.
doi: 10.1016/j.pcad.2013.10.003 |
| 19 |
Abizanda P , Navarro JL , García-Tomás MI , et al. Validity and usefulness of hand-held dynamometry for measuring muscle strength in community-dwelling older persons[J]. Arch Gerontol Geriatr, 2012, 54 (1): 21- 27.
doi: 10.1016/j.archger.2011.02.006 |
| 20 |
Chen LK , Woo J , Assantachai P , et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment[J]. J Am Med Dir Assoc, 2020, 21 (3): 300- 307.
doi: 10.1016/j.jamda.2019.12.012 |
| 21 |
Donini LM , Busetto L , Bischoff SC , et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement[J]. Clin Nutr, 2022, 41 (4): 990- 1000.
doi: 10.1016/j.clnu.2021.11.014 |
| 22 |
Evans WJ , Morley JE , Argilés J , et al. Cachexia: A new definition[J]. Clin Nutr, 2008, 27 (6): 793- 799.
doi: 10.1016/j.clnu.2008.06.013 |
| 23 | Li TH , Chang YS , Liu CW , et al. The prevalence and risk factors of sarcopenia in rheumatoid arthritis patients: A systematic review and meta-regression analysis[J]. Semin Arthritis Rheu, 2020, 51 (1): 236- 245. |
| 24 |
Challal S , Minichiello E , Boissier MC , et al. Cachexia and adiposity in rheumatoid arthritis. Relevance for disease management and clinical outcomes[J]. Joint Bone Spine, 2016, 83 (2): 127- 133.
doi: 10.1016/j.jbspin.2015.04.010 |
| 25 |
Tian P , Xiong J , Wu W , et al. Impact of the malnutrition on mortality in rheumatoid arthritis patients: A cohort study from NHANES 1999-2014[J]. Front Nutr, 2023, 9, 993061.
doi: 10.3389/fnut.2022.993061 |
| 26 |
Rall LC , Roubenoff R . Rheumatoid cachexia: Metabolic abnormalities, mechanisms and interventions[J]. Rheumatology, 2004, 43 (10): 1219- 1223.
doi: 10.1093/rheumatology/keh321 |
| 27 |
Jutley GS , Sahota K , Sahbudin I , et al. Relationship between inflammation and metabolism in patients with newly presenting rheumatoid arthritis[J]. Front Immunol, 2021, 12, 676105.
doi: 10.3389/fimmu.2021.676105 |
| 28 |
Cano-García L , Manrique-Arija S , Domínguez-Quesada C , et al. Sarcopenia and nutrition in elderly rheumatoid arthritis patients: A cross-sectional study to determine prevalence and risk factors[J]. Nutrients, 2023, 15 (11): 2440.
doi: 10.3390/nu15112440 |
| 29 |
Pan J , Wu T , Ma JD , et al. Geriatric nutrition risk index: A more powerful index identifying muscle mass loss in patients with rheumatoid arthritis[J]. Clin Rheumatol, 2024, 43 (4): 1299- 1310.
doi: 10.1007/s10067-024-06918-3 |
| 30 |
Engvall IL , Elkan AC , Tengstrand B , et al. Cachexia in rheumatoid arthritis is associated with inflammatory activity, physical dis-ability, and low bioavailable insulin-like growth factor[J]. Scand J Rheumatol, 2008, 37 (5): 321- 328.
doi: 10.1080/03009740802055984 |
| 31 |
Baker JF , Giles JT , Weber D , et al. Sarcopenic obesity in rheumatoid arthritis: Prevalence and impact on physical functioning[J]. Rheumatology (Oxford), 2022, 61 (6): 2285- 2294.
doi: 10.1093/rheumatology/keab710 |
| 32 |
Pan J , Zou YW , Zhu YY , et al. Muscle mass loss is associated with physical dysfunction in patients with early rheumatoid arthritis[J]. Front Nutr, 2022, 9, 1007184.
doi: 10.3389/fnut.2022.1007184 |
| 33 |
Dent E , Morley JE , Cruz-Jentoft AJ , et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, diagnosis and management[J]. J Nutr Health Aging, 2018, 22 (10): 1148- 1161.
doi: 10.1007/s12603-018-1139-9 |
| 34 | Liao CD , Chen HC , Huang SW , et al. Exercise therapy for sarcopenia in rheumatoid arthritis: A meta-analysis and meta-regression of randomized controlled trials[J]. Clin Rehabil, 2002, 36 (2): 145- 157. |
| 35 |
Rausch Osthoff AK , Juhl CB , Knittle K , et al. Effects of exercise and physical activity promotion: Meta-analysis informing the 2018 EULAR recommendations for physical activity in people with rheumatoid arthritis, spondyloarthritis and hip/knee osteoarthritis[J]. RMD Open, 2018, 4 (2): e000713.
doi: 10.1136/rmdopen-2018-000713 |
| 36 |
Deutz NE , Bauer JM , Barazzoni R , et al. Protein intake and exercise for optimal muscle function with aging: Recommendations from the ESPEN Expert Group[J]. Clin Nutr, 2014, 33 (6): 929- 936.
doi: 10.1016/j.clnu.2014.04.007 |
| 37 |
Groen BB , Horstman AM , Hamer HM , et al. Post-prandial protein handling: You are what you just ate[J]. PLoS One, 2015, 10 (11): e0141582.
doi: 10.1371/journal.pone.0141582 |
| 38 |
De Spiegeleer A , Beckwée D , Bautmans I , et al. Sarcopenia Guidelines Development group of the Belgian Society of Gerontology and Geriatrics (BSGG). Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: An umbrella review of systematic reviews and meta-analyses[J]. Drugs Aging, 2018, 35 (8): 719- 734.
doi: 10.1007/s40266-018-0566-y |
| [1] | Doudou MA, Zhemin LU, Qian GUO, Sha ZHU, Jin GU, Yan DING, Lianjie SHI. Successful treatment of rheumatoid arthritis complicated with myasthenia gravis with low-dose rituximab: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1110-1114. |
| [2] | Rui YAN, Dan KE, Yan ZHANG, Li LI, Huanran SU, Wei CHEN, Mingxia SUN, Xiaomin LIU, Liang LUO. Diagnostic significance of serum chemokine CXCL-10 and Krebs von den lungen-6 level in patients with rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 956-962. |
| [3] | Liang ZHAO, Chenglong SHI, Ke MA, Jing ZHAO, Xiao WANG, Xiaoyan XING, Wanxing MO, Yirui LIAN, Chao GAO, Yuhui LI. Immunological characteristics of patients with anti-synthetase syndrome overlap with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 972-979. |
| [4] | Yijun HAN, Xiaoli CHEN, Changhong LI, Jinxia ZHAO. Application status of methotrexate in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 994-1000. |
| [5] | Dongwu LIU, Jie CHEN, Mingli GAO, Jing YU. Rheumatoid arthritis with Castleman-like histopathology in lymph nodes: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 928-931. |
| [6] | Huina HUANG,Jing ZHAO,Xiangge ZHAO,Ziran BAI,Xia LI,Guan WANG. Regulatory effect of lactate on peripheral blood CD4+ T cell subsets in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 519-525. |
| [7] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [8] | Xue ZOU,Xiao-juan BAI,Li-qing ZHANG. Effectiveness of tofacitinib combined with iguratimod in the treatment of difficult-to-treat moderate-to-severe rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1013-1021. |
| [9] | Qi WU,Yue-ming CAI,Juan HE,Wen-di HUANG,Qing-wen WANG. Correlation between dyslipidemia and rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 982-992. |
| [10] | Jing-feng ZHANG,Yin-ji JIN,Hui WEI,Zhong-qiang YAO,Jin-xia ZHAO. Correlation analysis between body mass index and clinical characteristics of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 993-999. |
| [11] | Xiao-jin YAN,Yun-fei LIU,Ning MA,Jia-jia DANG,Jing-shu ZHANG,Pan-liang ZHONG,Pei-jin HU,Yi SONG,Jun MA. Assessment of prevalence of malnutrition among Chinese primary and secondary school students and analysis of policy effect during the period of the Program for the Development of Chinese Children 2011-2020 [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 593-599. |
| [12] | Yin-ji JIN,Lin SUN,Jin-xia ZHAO,Xiang-yuan LIU. Significance of IgA isotype of anti-v-raf murine sarcoma viral oncogene homologue B1 antibody in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 631-635. |
| [13] | Wen-xin CAI,Shi-cheng LI,Yi-ming LIU,Ru-yu LIANG,Jing LI,Jian-ping GUO,Fan-lei HU,Xiao-lin SUN,Chun LI,Xu LIU,Hua YE,Li-zong DENG,Ru LI,Zhan-guo LI. A cross-sectional study on the clinical phenotypes of rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1068-1073. |
| [14] | Fang CHENG,Shao-ying YANG,Xing-xing FANG,Xuan WANG,Fu-tao ZHAO. Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1074-1078. |
| [15] | Rui LIU,Jin-xia ZHAO,Liang YAN. Clinical characteristics of patients with rheumatoid arthritis complicated with venous thrombosis of lower extremities [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1079-1085. |
|
||