Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (6): 980-986. doi: 10.19723/j.issn.1671-167X.2024.06.006
Previous Articles Next Articles
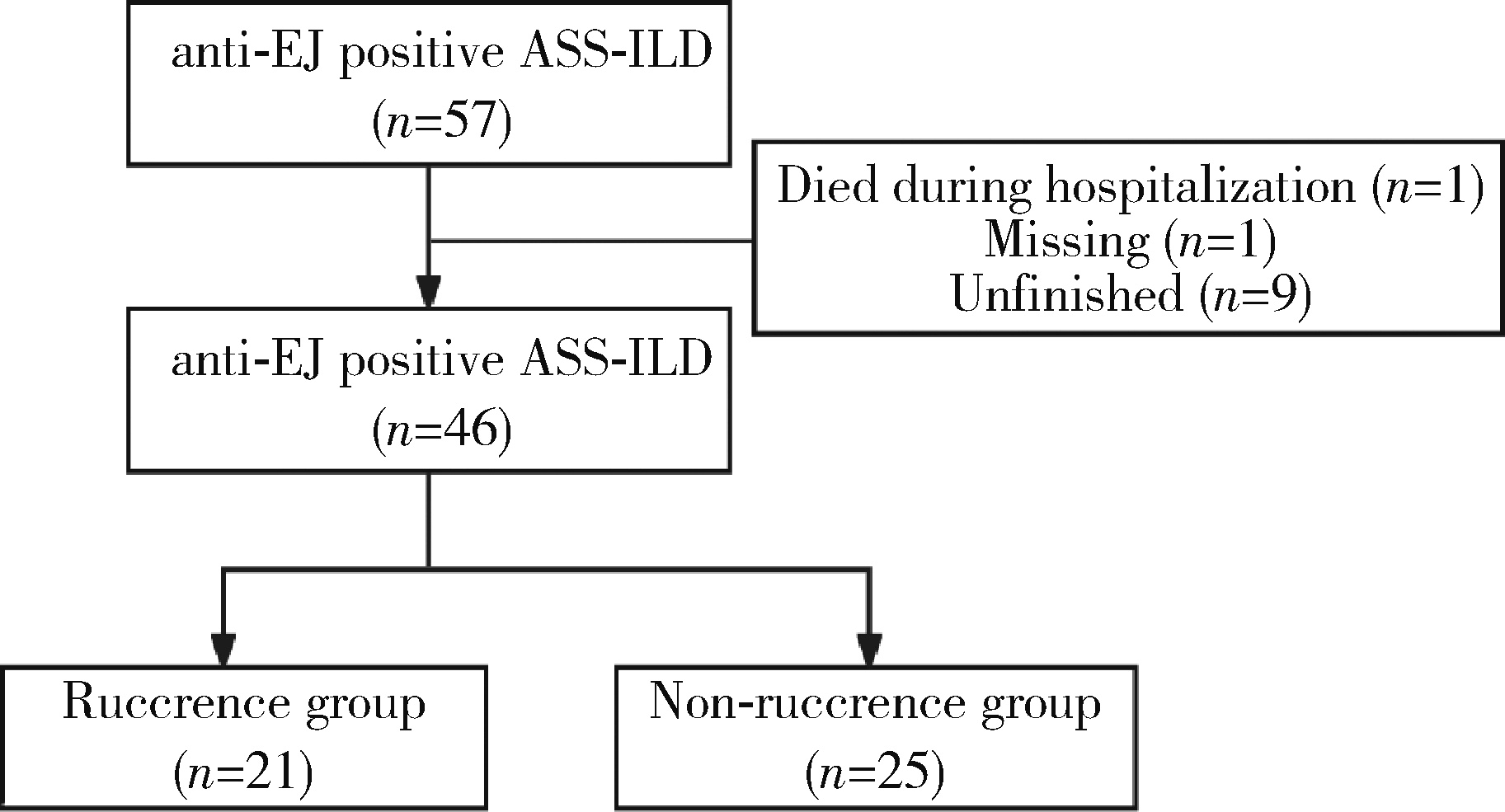
Analysis of clinical features of ruccrent interstitial lung disease in patients with anti-EJ positive antisynthetase syndrome
Yujing ZHU, Lei WANG, Chengyin LYU, Wenfeng TAN, Miaojia ZHANG*( )
)
- Department of Rheumatology and Immunology, the First Affiliated Hospital of Nanjing Medical University, Nanjing 210029, China
CLC Number:
- R563
| 1 |
Aggarwal R , Cassidy E , Fertig N , et al. Patients with non-Jo-1 anti-tRNA synthetase autoantibodies have worse survival than Jo-1 positive patients[J]. Ann Rheum Dis, 2014, 73 (1): 227- 232.
doi: 10.1136/annrheumdis-2012-201800 |
| 2 | Sreevilasan SK , Devarasetti P , Narahari NK , et al. Clinical profile and treatment outcomes in antisynthetase syndrome: A tertiary centre experience[J]. Rheumatol Adv Pract, 2021, 5 (Suppl 2): ii10- ii18. |
| 3 |
Hamaguchi Y , Fujimoto M , Matsushita T , et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: Heterogeneity within the syndrome[J]. PLoS One, 2013, 8 (4): e60442.
doi: 10.1371/journal.pone.0060442 |
| 4 |
Teel A , Lu J , Park J , et al. The role of myositis-specific autoantibodies and the management of interstitial lung disease in idiopathic inflammatory myopathies: A systematic review[J]. Semin Arthritis Rheum, 2022, 57, 152088.
doi: 10.1016/j.semarthrit.2022.152088 |
| 5 |
Zhang Y , Ge Y , Yang H , et al. Clinical features and outcomes of the patients with anti-glycyl tRNA synthetase syndrome[J]. Clin Rheumatol, 2020, 39 (8): 2417- 2424.
doi: 10.1007/s10067-020-04979-8 |
| 6 |
Watanabe K , Handa T , Tanizawa K , et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias[J]. Respir Med, 2011, 105 (8): 1238- 1247.
doi: 10.1016/j.rmed.2011.03.022 |
| 7 |
Sasano H , Hagiwara E , Kitamura H , et al. Long-term clinical course of anti-glycyl tRNA synthetase (anti-EJ) antibody-related interstitial lung disease pathologically proven by surgical lung biopsy[J]. BMC Pulm Med, 2016, 16 (1): 168.
doi: 10.1186/s12890-016-0325-y |
| 8 |
Hozumi H , Fujisawa T , Nakashima R , et al. Efficacy of glucocorticoids and calcineurin inhibitors for anti-aminoacyl-trna synthetase antibody-positive polymyositis/dermatomyositis-associated interstitial lung disease: A propensity score-matched analysis[J]. J Rheumatol, 2019, 46 (5): 509- 517.
doi: 10.3899/jrheum.180778 |
| 9 | Yorishima Y , Tominaga M , Fujimoto K , et al. Combination of prednisolone and calcineurin inhibitors prevents lung function decline in patients with anti-aminoacyl-tRNA synthetase antibody-positive polymyositis/dermatomyositis[J]. Kurume Med J, 2023, 69 (1/2): 19- 30. |
| 10 |
Martínez-García EA , Lujano-Benítez AV , Gercía-De La Torre Ⅰ , et al. Good response to mycophenolate mofetil on treatment of interstitial lung disease in polymyositis associated with antisynthetase syndrome positive for anti-EJ and anti-Ro52 antibodies[J]. Clin Rheumatol, 2020, 39 (9): 2837- 2839.
doi: 10.1007/s10067-020-05075-7 |
| 11 |
Langlois V , Gillibert A , Uzunhan Y , et al. Rituximab and cyclophosphamide in antisynthetase syndrome-related interstitial lung disease: An observational retrospective study[J]. J Rheumatol, 2020, 47 (11): 1678- 1686.
doi: 10.3899/jrheum.190505 |
| 12 |
Liu Y , Liu X , Xie M , et al. Clinical characteristics of patients with anti-EJ antisynthetase syndrome associated interstitial lung disease and literature review[J]. Respir Med, 2020, 165, 105920.
doi: 10.1016/j.rmed.2020.105920 |
| 13 |
Connors GR , Christopher-Stine L , Oddis CV , et al. Interstitial lung disease associated with the idiopathic inflammatory myopathies: What progress has been made in thepast 35 years[J]. Chest, 2010, 138 (6): 1464- 1474.
doi: 10.1378/chest.10-0180 |
| 14 |
Bohan A , Peter JB . Polymyositis and dermatomyositis (first of two parts)[J]. N Engl J Med, 1975, 292 (7): 344- 347.
doi: 10.1056/NEJM197502132920706 |
| 15 |
Raghu G , Remy-Jardin M , Richeldi L , et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS /ALAT clinical practice guideline[J]. Am J Respir Crit Care Med, 2022, 205 (9): e18- e47.
doi: 10.1164/rccm.202202-0399ST |
| 16 |
Targoff IN . Autoantibodies to aminoacyl-transfer RNA synthetases for isoleucine and glycine. Two additional synthetases are antigenic in myositis[J]. J Immunol, 1990, 144 (5): 1737- 1743.
doi: 10.4049/jimmunol.144.5.1737 |
| 17 | Tang HS, Tang IYK, Ho RTC, et al. Clinical heterogeneity and prognostic factors of anti-synthetase syndrome: A multi-centered retrospective cohort study[J]. Rheumatology (Oxford), 2023 (2023-12-14)[2024-09-01]. https://Doi.org/10.1093/rheumatology/kead671. |
| 18 |
García-Bravo L , Calle-Rubio M , Fernández-Arquero M , et al. Association of anti-SARS-COV-2 vaccine with increased incidence of myositis-related anti-RNA-synthetases auto-antibodies[J]. J Transl Autoimmun, 2022, 5, 100160.
doi: 10.1016/j.jtauto.2022.100160 |
| 19 | Irie Y , Wakabayashi H , Matuzawa Y , et al. A case of anti-synthetase syndrome with anti-glycyl tRNA synthetases antibody de-veloped after COVID-19[J]. Cureus, 2024, 16 (4): e58004. |
| 20 |
Shimizu H , Matsumoto H , Sasajima T , et al. New-onset dermatomyositis following COVID-19: A case report[J]. Front Immunol, 2022, 13, 1002329.
doi: 10.3389/fimmu.2022.1002329 |
| 21 | Peña C , Kalara N , Velagapudi P . A case of antisynthetase syndrome in the setting of SARS-Cov-2 infection[J]. Cureus, 2023, 15 (6): e40588. |
| 22 |
Elsayed M , Abdelgabar A , Karmani J , et al. A case of antisynthetase syndrome initially presented with interstitial lung disease mimicking COVID-19[J]. J Med Cases, 2023, 14 (1): 25- 30.
doi: 10.14740/jmc4031 |
| 23 | Tranah E , MacBrayne A , Bhadauria N , et al. A case of antisynthetase syndrome presenting solely with life-threatening interstitial lung disease[J]. Clin Med (Lond), 2023, 23 (1): 85- 87. |
| 24 | 周云, 吕成银, 尤含笑, 等. 不同抗体亚型抗合成酶综合征并发间质性肺疾病临床特征分析[J]. 中华风湿病学杂志, 2024, 28 (8): 538- 544. |
| [1] | Yukai LI, Hongyan WANG, Liang LUO, Yun LI, Chun LI. Clinical significance of antiphospholipid antibodies in Behcet disease with thrombosis [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1036-1040. |
| [2] | Rui YAN, Dan KE, Yan ZHANG, Li LI, Huanran SU, Wei CHEN, Mingxia SUN, Xiaomin LIU, Liang LUO. Diagnostic significance of serum chemokine CXCL-10 and Krebs von den lungen-6 level in patients with rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 956-962. |
| [3] | Yuqing LI,Biao WANG,Peng QIAO,Wei WANG,Xing GUAN. Medium to long-term efficacy of tension-free vaginal tape procedure in the treatment of female recurrent stress urinary incontinence [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 600-604. |
| [4] | Zhanhong LAI,Jiachen LI,Zelin YUN,Yonggang ZHANG,Hao ZHANG,Xiaoyan XING,Miao SHAO,Yuebo JIN,Naidi WANG,Yimin LI,Yuhui LI,Zhanguo LI. A unicenter real-world study of the correlation factors for complete clinical response in idiopathic inflammatory myopathies [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 284-292. |
| [5] | Qi WU,Yue-ming CAI,Juan HE,Wen-di HUANG,Qing-wen WANG. Correlation between dyslipidemia and rheumatoid arthritis associated interstitial lung disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 982-992. |
| [6] | Wen-gen LI,Xiao-dong GU,Rui-qiang WENG,Su-dong LIU,Chao CHEN. Expression and clinical significance of plasma exosomal miR-34-5p and miR-142-3p in systemic sclerosis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1022-1027. |
| [7] | Chieko MORIMOTO,Yi-qin WANG,Rong ZHOU,Jian-liu WANG. Clinical analysis of fertility-sparing therapy of patients with complex atypical hyperplasia and endometrial cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 936-942. |
| [8] | Yue WANG,Shuang ZHANG,Hong ZHANG,Li LIANG,Ling XU,Yuan-jia CHENG,Xue-ning DUAN,Yin-hua LIU,Ting LI. Clinicopathological features and prognosis of hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 853-862. |
| [9] | Su-hua CHEN,Jun YANG,Xin CHEN,Chen-long YANG,Jian-jun SUN,Guo-zhong LIN,Tao YU,Xin YANG,Yun-feng HAN,Chao WU,Yu SI,Kai-ming MA. Surgical treatment of large and giant recurrent meningiomas near the middle and posterior third part of the superior sagittal sinus with extracranial invading [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 1006-1012. |
| [10] | ZHANG Pu-li,YANG Hong-xia,ZHANG Li-ning,GE Yong-peng,PENG Qing-lin,WANG Guo-chun,LU Xin. Value of serum YKL-40 in the diagnosis of anti-MDA5-positive patients with dermatomyositis complicated with severe pulmonary injury [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1055-1060. |
| [11] | LIU Lei,QIN Yan-chun,WANG Guo-liang,ZHANG Shu-dong,HOU Xiao-fei,MA Lu-lin. Strategy of reoperation for pheochromocytoma and paraganglioma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 793-797. |
| [12] | XIA Fang-fang,LU Fu-ai,LV Hui-min,YANG Guo-an,LIU Yuan. Clinical characteristics and related factors of systemic lupus erythematosus with interstitial pneumonia [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 266-272. |
| [13] | Huan-bin YU,Wen-jie WU,Xiao-ming LV,Yan SHI,Lei ZHENG,Jian-guo ZHANG. 125I seed brachytherapy for recurrent salivary gland carcinoma after external radiotherapy [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 919-923. |
| [14] | Xu-chu ZHANG,Jian-hua ZHANG,Rong-fu WANG,Yan FAN,Zhan-li FU,Ping YAN,Guang-yu ZHAO,Yan-xia BAI. Diagnostic value of 18F-FDG PET/CT and tumor markers (CEA, CA19-9, CA24-2) in recurrence and metastasis of postoperative colorectal moderately differentiated adenocarcinoma [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1071-1077. |
| [15] | Qi TANG,Rong-cheng LIN,Lin YAO,Zheng ZHANG,Han HAO,Cui-jian ZHANG,Lin CAI,Xue-song LI,Zhi-song HE,Li-qun ZHOU. Clinicopathologic features and prognostic analyses of locally recurrent renal cell carcinoma patients after initial surgery [J]. Journal of Peking University(Health Sciences), 2019, 51(4): 628-631. |
|
||