北京大学学报(医学版) ›› 2024, Vol. 56 ›› Issue (2): 213-222. doi: 10.19723/j.issn.1671-167X.2024.02.003
基于胚胎干细胞模型的Cry1Ab蛋白发育毒性
- 1. 北京大学公共卫生学院营养与食品卫生学系, 北京 100191
2. 食品安全毒理学研究与评价北京市重点实验室,北京 100191
Developmental toxicity of Cry1Ab protein in the embryonic stem-cell model
Yuanzhi JIAN1,Fei WANG1,Ning YIN1,Ruoyu ZHOU1,Junbo WANG1,2,*( )
)
- 1. Department of Nutrition and Food Hygiene, School of Public Health, Peking University, Beijing 100191, China
2. Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety, Beijing 100191, China
摘要:
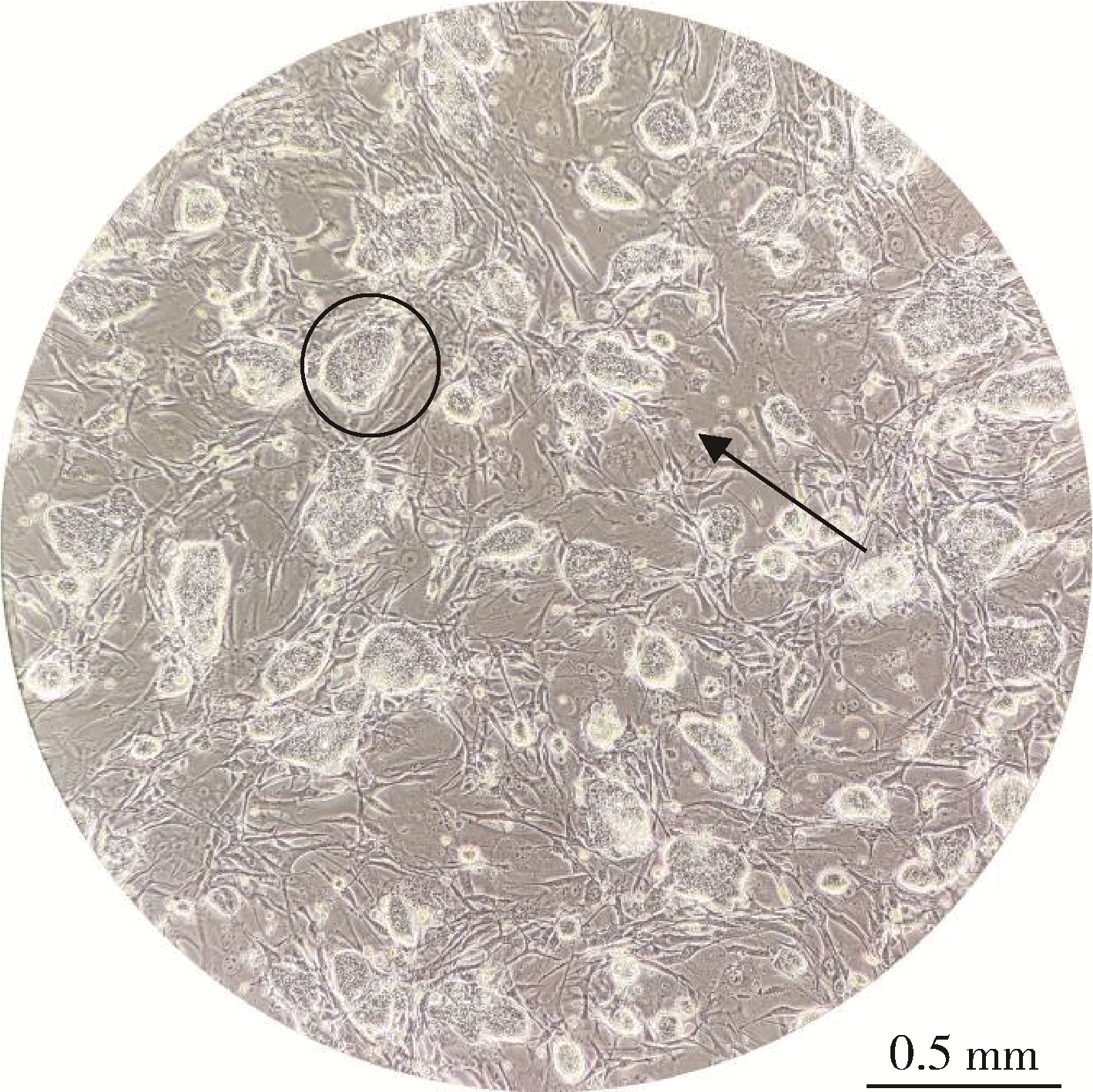
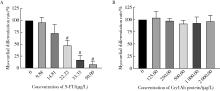
目的: 通过胚胎干细胞发育毒性评价模型研究Cry1Ab蛋白对于细胞增殖和分化能力的影响,以评估其发育毒性。方法: 设置Cry1Ab蛋白7个剂量组(31.25、62.50、125.00、250.00、320.00、1 000.00、2 000.00 μg/L),以5-氟尿嘧啶(5-fluorouracil,5-FU)为阳性对照,以磷酸缓冲盐溶液(phosphate buffer saline,PBS)为溶剂对照,分别处理小鼠胚胎干细胞D3(embryonic stem cell line D3,ES-D3)和小鼠成纤维细胞3T3。通过CCK-8法检测细胞活性,计算受试物对于不同细胞的增殖半抑制浓度(50% inhibition concentration of growth and viability,IC50)。设置Cry1Ab蛋白5个剂量组(125.00、250.00、320.00、1 000.00、2 000.00 μg/L),设置溶剂对照(PBS),同时以5-FU为受试物进行模型验证,分别处理细胞后,通过拟胚胎体(embryonic bodies,EBs)培养法诱导ES-D3分化出心肌细胞;镜下观察EBs生长情况并测量其第3天和第5天的直径,观察并记录同批次EBs分化出搏动心肌细胞的比例,计算受试物的心肌分化半抑制浓度(50% inhibition concentration of differentiation,ID50),根据发育毒性判别函数对受试物的胚胎发育毒性进行分类;收集培养终点的EBs样本,进行实时定量聚合酶链式反应(real-time quantitative po-lymerase chain reaction,qPCR),检测心肌分化相关标志物(Oct3/4、GATA-4、Nkx2.5和β-MHC)的mRNA表达情况。结果: 5-FU的IC50, 3T3为46.37 μg/L,IC50, ES为32.67 μg/L,ID50, ES为21.28 μg/L,根据判别函数结果将5-FU分类为强胚胎毒性物质。不同浓度的Cry1Ab蛋白处理组的3T3细胞和ES-D3细胞活性与对照组相比差异均无统计学意义(P>0.05)。与对照组相比,Cry1Ab蛋白处理组分化第3天和第5天的EBs直径差异无统计学意义(P>0.05),EBs形态也未见明显差异;不同浓度Cry1Ab蛋白处理组的心肌分化率与对照组相比差异无统计学意义(P>0.05)。5-FU使β-MHC、Nkx2.5和GATA-4的mRNA表达水平降低(P < 0.05),且具有剂量依赖趋势(P < 0.05),而与细胞多能性相关的标志物Oct3/4 mRNA表达水平则呈升高趋势(P < 0.05);Cry1Ab蛋白处理组的成熟心肌标志物β-MHC、心肌早期分化标志物Nkx2.5和GATA-4、多能性相关标志物Oct3/4的mRNA表达水平与对照组相比差异均无统计学意义(P>0.05)。结论: 本实验模型中未观察到31.25~2 000.00 μg/L的Cry1Ab蛋白具有发育毒性。
中图分类号:
- R155.5
| 1 |
SchnepfE,CrickmoreN,Van RieJ,et al.Bacillus thuringiensis and its pesticidal crystal proteins[J].Microbiol Mol Biol Rev,1998,62(3):775-806.
doi: 10.1128/MMBR.62.3.775-806.1998 |
| 2 |
Rubio-InfanteN,Moreno-FierrosL.An overview of the safety and biological effects of Bacillus thuringiensis Cry toxins in mammals[J].J Appl Toxicol,2016,36(5):630-648.
doi: 10.1002/jat.3252 |
| 3 | ÁlvarezF,MesséanA,StreisslF.Assessment of the 2019 post-market environmental monitoring report on the cultivation of genetically modified maize MON810 in the EU[J].EFSA J,2021,19(7):e06683. |
| 4 | KochMS,WardJM,LevineSL,et al.The food and environmental safety of Bt crops[J].Front Plant Sci,2015,6,283. |
| 5 | VieiraL,HissaDC,SouzaT,et al.Assessing the effects of an acute exposure to worst-case concentration of Cry proteins on zebrafish using the embryotoxicity test and proteomics analysis[J].Chemosphere,2021,264(Pt 2):128538. |
| 6 |
BøhnT,RoverCM,SemenchukPR.Daphnia magna negatively affected by chronic exposure to purified Cry-toxins[J].Food Chem Toxicol,2016,91,130-140.
doi: 10.1016/j.fct.2016.03.009 |
| 7 |
AlvesRDC,FerreiraCGM,Ferreira de MeloIM,et al.Renal and hepatic changes in the offspring of rats that received biological insecticides during pregnancy and lactation[J].Acta Histochem,2021,123(8):151799.
doi: 10.1016/j.acthis.2021.151799 |
| 8 |
MesnageR,ClairE,GressS,et al.Cytotoxicity on human cells of Cry1Ab and Cry1Ac Bt insecticidal toxins alone or with a glyphosate-based herbicide[J].J Appl Toxicol,2013,33(7):695-699.
doi: 10.1002/jat.2712 |
| 9 |
Mendoza-AlmanzaG,Rocha-ZavaletaL,Aguilar-ZacaríasC,et al.Cry1A Proteins are cytotoxic to HeLa but not to SiHa cervical cancer cells[J].Curr Pharm Biotechnol,2019,20(12):1018-1027.
doi: 10.2174/1389201020666190802114739 |
| 10 |
SoberónM,PortugalL,Garcia-GómezBI,et al.Cell lines as models for the study of Cry toxins from Bacillus thuringiensis[J].Insect Biochem Mol Biol,2018,93,66-78.
doi: 10.1016/j.ibmb.2017.12.008 |
| 11 |
GenschowE,SpielmannH,ScholzG,et al.Validation of the embryonic stem cell test in the international ECVAM validation study on three in vitro embryotoxicity tests[J].Altern Lab Anim,2004,32(3):209-244.
doi: 10.1177/026119290403200305 |
| 12 |
LiuH,RenC,LiuW,et al.Embryotoxicity estimation of commonly used compounds with embryonic stem cell test[J].Mol Med Rep,2017,16(1):263-271.
doi: 10.3892/mmr.2017.6552 |
| 13 | zur NiedenN I,RufL J,KempkaG,et al.Molecular markers in embryonic stem cells[J].Toxicol In Vitro,2001,15(4/5):455-461. |
| 14 | SpielmannH,PohlI,DöringB,et al.The embryonic stem cell test, an in vitro embryotoxicity test using two permanent mouse cell lines: 3T3 fibroblasts and embryonic stem cells[J].In Vitr Mol Toxicol,1997,10(1):119-127. |
| 15 | ECVAM. DB-ALM protocol n° 113: Embryonic stem cell test (EST)[G]. EURL ECVAM, 2010. https://jeodpp.jrc.ec.europa.eu/ftp/jrc-opendata/EURL-ECVAM/datasets/DBALM/LATEST/online/DBALM_docs/113_P_Embryonic%20Stem%20Cell%20Test.pdf. |
| 16 |
PesceM,ScholerHR.Oct-4: Gatekeeper in the beginnings of mammalian development[J].Stem Cells,2001,19(4):271-278.
doi: 10.1634/stemcells.19-4-271 |
| 17 |
CharronF,ParadisP,BronchainO,et al.Cooperative interaction between GATA-4 and GATA-6 regulates myocardial gene expression[J].Mol Cell Biol,1999,19(6):4355-4365.
doi: 10.1128/MCB.19.6.4355 |
| 18 | 卜萌萌. 中胚层细胞分化新功能基因的鉴定及功能研究[D]. 北京: 北京协和医学院, 2020. |
| 19 |
SchlutermanMK,KrysiakAE,KathiriyaIS,et al.Screening and biochemical analysis of GATA4 sequence variations identified in patients with congenital heart disease[J].Am J Med Genet A,2007,143A(8):817-823.
doi: 10.1002/ajmg.a.31652 |
| 20 |
zur NiedenNI,KempkaG,AhrHJ.Molecular multiple endpoint embryonic stem cell test: A possible approach to test for the teratogenic potential of compounds[J].Toxicol Appl Pharmacol,2004,194(3):257-269.
doi: 10.1016/j.taap.2003.09.019 |
| 21 |
GordeevaO,GordeevA.Comparative assessment of toxic responses in 3D embryoid body differentiation model and mouse early embryos treated with 5-hydroxytryptophan[J].Arch Toxicol,2021,95(1):253-269.
doi: 10.1007/s00204-020-02909-w |
| 22 |
LiangS,ZhouH,YinN,et al.Embryoid body-based RNA-seq analyses reveal a potential TBBPA multifaceted developmental toxicity[J].J Hazard Mater,2019,376,223-232.
doi: 10.1016/j.jhazmat.2019.05.030 |
| 23 |
KimI Q,MarikawaY.Embryoid body test with morphological and molecular endpoints implicates potential developmental toxicity of trans-resveratrol[J].Toxicol Appl Pharmacol,2018,355,211-225.
doi: 10.1016/j.taap.2018.07.006 |
| 24 |
LeeJH,ParkSY,AhnC,et al.Pre-validation study of alternative developmental toxicity test using mouse embryonic stem cell-derived embryoid bodies[J].Food Chem Toxicol,2019,123,50-56.
doi: 10.1016/j.fct.2018.10.044 |
| 25 | LeeJH,ParkSY,AhnC,et al.Second-phase validation study of an alternative developmental toxicity test using mouse embryonic stem cell-derived embryoid bodies[J].J Physiol Pharmacol,2020,71(2):223-233. |
| 26 |
夏荃,鲍倩,蒋德菊,等.基于小鼠胚胎干细胞实验模型评价川芎水煎液的胚胎毒性[J].中成药,2018,40(9):1910-1915.
doi: 10.3969/j.issn.1001-1528.2018.09.003 |
| 27 |
van OostromCT,SlobW,van der VenLT.Defining embryonic developmental effects of chemical mixtures using the embryonic stem cell test[J].Food Chem Toxicol,2020,140,111284.
doi: 10.1016/j.fct.2020.111284 |
| 28 |
ArisA,LeblancS.Maternal and fetal exposure to pesticides associated to genetically modified foods in Eastern Townships of Quebec, Canada[J].Reprod Toxicol,2011,31(4):528-533.
doi: 10.1016/j.reprotox.2011.02.004 |
| 29 |
WalshMC,BuzoianuSG,ReaMC,et al.Effects of feeding Bt MON810 maize to pigs for 110 days on peripheral immune response and digestive fate of the cry1Ab gene and truncated Bt toxin[J].PLoS One,2012,7(5):e36141.
doi: 10.1371/journal.pone.0036141 |
| 30 |
ShimadaN,KimYS,MiyamotoK,et al.Effects of Bacillus thuringiensis Cry1Ab toxin on mammalian cells[J].J Vet Med Sci,2003,65(2):187-191.
doi: 10.1292/jvms.65.187 |
| 31 |
BondzioA,StumpffF,SchönJ,et al.Impact of Bacillus thurin-giensis toxin Cry1Ab on rumen epithelial cells (REC): A new in vitro model for safety assessment of recombinant food compounds[J].Food Chem Toxicol,2008,46(6):1976-1984.
doi: 10.1016/j.fct.2008.01.038 |
| 32 |
BondzioA,LodemannU,WeiseC,et al.Cry1Ab treatment has no effects on viability of cultured porcine intestinal cells, but triggers Hsp70 expression[J].PLoS One,2013,8(7):e67079.
doi: 10.1371/journal.pone.0067079 |
| 33 | 郭梦凡,韩超,李岩,等.转Cry1Ab和epsps基因抗虫耐除草剂玉米对三代繁殖大鼠子代的神经行为与认知能力的影响[J].卫生研究,2018,47(3):419-424. |
| 34 |
LiZ,GaoY,ZhangM,et al.Effects of a diet containing genetically modified rice expressing the Cry1Ab/1Ac protein (Bacillus thuringiensis toxin) on broiler chickens[J].Arch Anim Nutr,2015,69(6):487-498.
doi: 10.1080/1745039X.2015.1087749 |
| 35 |
BuzoianuSG,WalshMC,ReaMC,et al.Transgenerational effects of feeding genetically modified maize to nulliparous sows and offspring on offspring growth and health[J].J Anim Sci,2013,91(1):318-330.
doi: 10.2527/jas.2012-5360 |
| 36 | RidingsJE.The thalidomide disaster, lessons from the past[J].Methods Mol Biol,2013,947,575-586. |
| 37 |
NiraulaPM,FondongVN.Development and adoption of geneti-cally engineered plants for virus resistance: Advances, opportunities and challenges[J].Plants,2021,10(11):2339.
doi: 10.3390/plants10112339 |
| [1] | 潘媛,顾航,肖涵,赵笠君,汤祎熳,葛雯姝. 泛素特异性蛋白酶42调节人脂肪干细胞成骨向分化[J]. 北京大学学报(医学版), 2024, 56(1): 9-16. |
| [2] | 张胜男,安娜,欧阳翔英,刘颖君,王雪奎. 生长停滞特异性蛋白6在人牙周膜细胞迁移及成骨分化中的作用[J]. 北京大学学报(医学版), 2021, 53(1): 9-15. |
| [3] | 谢静,赵玉鸣,饶南荃,汪晓彤,方滕姣子,李晓霞,翟越,李静芝,葛立宏,王媛媛. 3种口腔颌面部来源的间充质干细胞成血管内皮分化潜能的比较研究[J]. 北京大学学报(医学版), 2019, 51(5): 900-906. |
| [4] | 隋华欣,吕培军,王勇,冯驭驰. 低能量激光照射对人脂肪来源干细胞/海藻酸钠/明胶三维生物打印体成骨能力的影响[J]. 北京大学学报(医学版), 2018, 50(5): 868-875. |
| [5] | 隋华欣, 吕培军, 王宇光, 王勇, 孙玉春. 低能量激光照射对人脂肪基质细胞增殖分化的影响[J]. 北京大学学报(医学版), 2017, 49(2): 337-343. |
| [6] | 凌龙,赵玉鸣,葛立宏. 不同炎症状态下犬年轻恒牙牙髓干细胞增殖及成骨分化能力的改变[J]. 北京大学学报(医学版), 2016, 48(5): 878-883. |
| [7] | 俞光岩, 曹彤, 邹晓晖, 张学慧, 傅歆, 彭双清, 邓旭亮, 李盛林, 刘鹤, 肖苒, 欧阳宏伟, 彭晖, 陈晓, 赵增明,王晓颖……. 基于人胚胎干细胞健康安全评价体系的构建[J]. 北京大学学报(医学版), 2016, 48(1): 1-4. |
| [8] | 张晓,刘云松,吕珑薇,陈彤,吴刚,周永胜. 骨形态发生蛋白2/7异二聚体对人脂肪间充质干细胞成骨分化的促进作用[J]. 北京大学学报(医学版), 2016, 48(1): 37-44. |
| [9] | 葛雯姝,汤祎熳,张晓,刘云松,周永胜. 构建一种可评价脂肪间充质干细胞成骨向分化的荧光素酶报告系统[J]. 北京大学学报(医学版), 2016, 48(1): 170-174. |
| [10] | 王晓飞,吕培军,宋杨,王勇,孙玉春 . 短时CaCl2刺激对人脂肪干细胞增殖与成骨向分化的影响[J]. 北京大学学报(医学版), 2015, 47(6): 971-976. |
| [11] | 贾双双,李伟阳,刘欣,李丽英. 转化生长因子-β1 通过产生活性氧诱导骨髓间充质干细胞分化为肌成纤维细胞[J]. 北京大学学报(医学版), 2015, 47(5): 737-742. |
| [12] | 宋一萌, 马潞林, 李肖霞, 朱毅. 前列腺素E2促骨髓来源内皮前体细胞分化的机制[J]. 北京大学学报(医学版), 2015, 47(4): 577-581. |
| [13] | 任玉兰, 战园, 路璐, 李盛林, 傅歆, 俞光岩, 曹彤, 刘鹤. 人胚胎干细胞分化为角质形成细胞过程中标志物的表达特点[J]. 北京大学学报(医学版), 2015, 47(2): 305-311. |
| [14] | 许向亮,王恩博,崔念晖. 脱细胞真皮基质对年轻恒牙牙乳头干细胞分化的作用[J]. 北京大学学报(医学版), 2014, 46(1): 12-18. |
| [15] | 丁茜,张凤秋,马玉实. 淫羊藿苷对人牙周膜细胞骨向分化基因表达的影响[J]. 北京大学学报(医学版), 2013, 45(6): 975-978. |
|
||