北京大学学报(医学版) ›› 2018, Vol. 50 ›› Issue (6): 962-967. doi: 10.19723/j.issn.1671-167X.2018.06.004
去整合素金属蛋白酶对成骨分化的影响
刘霞1,李英妮2,孙晓麟2,彭清林1,卢昕1,王国春1,△( )
)
- 1. 中日友好医院风湿免疫科, 北京 100029
2. 北京大学人民医院风湿免疫科, 北京 100044
Effects of integrin metalloproteinases on osteogenic differentiation
Xia LIU1,Ying ni LI2,Xiao li SUN2,Qing lin PENG1,Xin LU1,Guo chun WANG1,△( )
)
- 1. Department of Rheumatology and Immunology, China?Japan Friendship Hospital, Beijing 100029, China
2. Department of Rheumatology and Immunology, Peking University People’s Hospital, Beijing 100044, China
摘要:
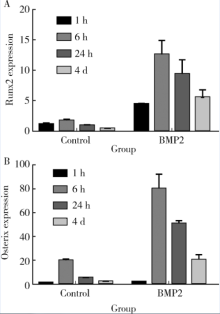
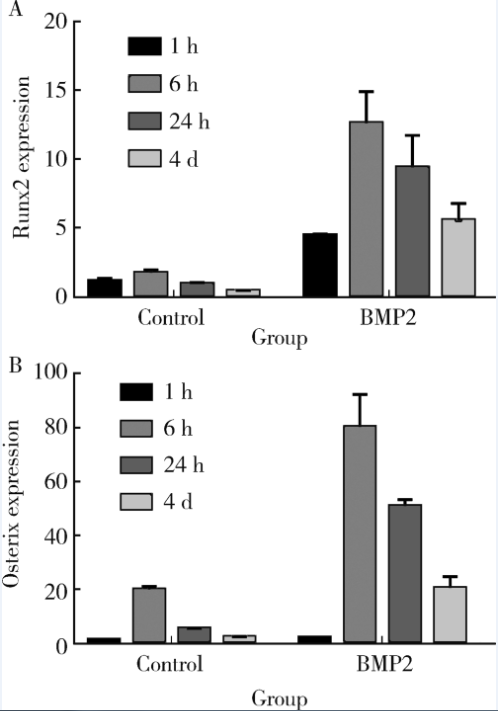
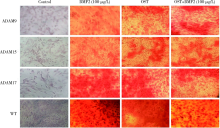
目的: 探讨去整合素金属蛋白酶(a disintegrin and metalloproteinase, ADAM)9、15、17在骨髓间充质干细胞(bone marrow mesenchymal stem cells,BMMSCs)成骨分化中的作用。方法:分离ADAM9、ADAM15、ADAM17的条件基因敲除小鼠及野生型(wild type,WT)小鼠的BMMSCs,培养刺激其分化为成骨细胞。比色法测定成骨标志碱性磷酸酶(alkaline phosphatase,ALP)活性,实时荧光定量PCR检测成骨早期转录因子Runx、Osterix,茜素红染色分析成骨矿物质形成情况。 结果:在无刺激的对照培养下,ADAM9组(8.08±0.34)、ADAM15组(6.46±3.40)、ADAM17(9.30±2.30)组的ALP活性与WT组(9.44±2.50)相比差异无统计学意义(P均>0.05)。用骨形态发生蛋白质2(bone morphogenic protein 2, BMP2)刺激培养, ADAM9组(14.22±3.25)、ADAM15组(10.14±2.40)的ALP均小于WT组(20.89±3.40), 差异有统计学意义(P均<0.05),而ADAM17组(23.56±2.50)虽大于WT组(20.89±3.40),但差异无统计学意义(P>0.05)。用成骨培养基(osteogenic induction medium, OST)刺激培养,ADAM9组(9.63±1.00)、ADAM15组(7.75±1.30)的ALP均小于WT组(12.97±1.30), 而ADAM17组(20.09±1.68)大于WT组(12.97±1.30),差异均有统计学意义(P均<0.05)。同样,BMP2与OST联合刺激培养后检测ALP,ADAM9组(15.75±1.30)、ADAM15组(12.43±1.30)均小于WT组(26.15±1.50),而ADAM17组(29.55±2.10)大于WT组,差异均有统计学意义(P均<0.05)。Runx2表达,在无刺激的对照培养下,ADAM9组(2.02±0.24)、ADAM15组(3.09±0.19)、ADAM17组(3.89±0.91)分别与WT组(2.02±0.21)相比差异均无统计学意义(P均>0.05);BMP2刺激培养后ADAM9组(7.00±0.23)、ADAM15组(6.04±0.23)均小于WT组(12.6±0.23),而ADAM17组(18.52±1.39)大于WT组,差异均有统计学意义(P均<0.05)。Osterix表达,在无刺激的对照培养下,ADAM9组(9.60±3.87)、ADAM17组(12.4±3.00)与WT组(10.9±1.10)比较差异均无统计学意义(P均>0.05),但ADAM15组(6.50±1.51)低于WT组(10.9±1.10), 差异有统计学意义(P<0.05);BMP2刺激培养后ADAM9组(39.20±3.23)、ADAM15组(20.50±4.80)均小于WT组(60.3±5.93), 而ADAM17组(80.2±3.30)大于WT组,差异均有统计学意义(P均<0.05)。茜素红染色,无刺激组未见明显橘红色团块,BMP2、OST、OST+BMP2刺激培养条件下各实验组均可见局部钙化结节形成,但量化分析差异无统计学意义(P>0.05)。 结论:ADAM9、15、17参与了BMMSCs的成骨分化,为调节BMMSCs成骨分化提供了新靶点。
中图分类号:
- R593.2
| [1] |
Reiss K, Saftig P . The “A Disintegrin And Metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions[J]. Semin Cell Dev Biol, 2009,20(2):126-137.
doi: 10.1016/j.semcdb.2008.11.002 pmid: 19049889 |
| [2] |
Edwards DR, Handsley MM, Pennington CJ . The ADAM metalloproteinases[J]. Mol Aspects Med, 2008,29(5):258-289.
doi: 10.1016/j.mam.2008.08.001 |
| [3] |
Walkiewicz K, Nowakowska-Zajdel E, Kozieł P , et al. The role of some ADAM-proteins and activation of the insulin growth factor-related pathway in colorectal cancer[J]. Cent Eur J Immunol, 2018,43(1):109-113.
doi: 10.5114/ceji.2018.74881 |
| [4] |
Horowitz JD, Liu S . ADAM-15 and glycocalyx shedding: a new perspective on sepsis-related vasomotor dysfunction[J]. Cardiovasc Res, 2018,114(13):1694-1695.
doi: 10.1093/cvr/cvy199 |
| [5] |
Komiya K, Enomoto H, Inoki I , et al. Expression of ADAM15 in rheumatoid synovium: up-regulation by vascular endothelial growth factor and possible implications for angiogenesis[J]. Arthritis Res Ther, 2005,7(6):R1158-1173.
doi: 10.1186/ar1796 pmid: 16277668 |
| [6] |
Govoni KE, Amaar YG, Kramer A , et al. Regulation of insulin-like growth factor binding protein-5, four and a half lim-2, and a disintegrin and metalloprotease-9 expression in osteoblasts[J]. Growth Horm IGF Res, 2006,16(1):49-56.
doi: 10.1016/j.ghir.2005.10.001 pmid: 16311053 |
| [7] |
Karadag A, Zhou M, Croucher PI . ADAM-9 (MDC-9/meltrin-gamma), a member of the a disintegrin and metalloproteinase family, regulates myeloma-cell-induced interleukin-6 production in osteoblasts by direct interaction with the alpha(v) beta5 integrin[J]. Blood, 2006,107(8):3271-3278.
doi: 10.1182/blood-2005-09-3830 |
| [8] |
Ishii S, Isozaki T, Furuya H , et al. ADAM-17 is expressed on rheumatoid arthritis fibroblast-like synoviocytes and regulates proinflammatory mediator expression and monocyte adhesion[J]. Arthritis Res Ther, 2018,20(1):159.
doi: 10.1186/s13075-018-1657-1 |
| [9] |
Haxaire C, Hakobyan N, Pannellini T , et al. Blood-induced bone loss in murine hemophilic arthropathy is prevented by blocking the iRhom2/ADAM17/TNFα pathway[J]. Blood, 2018,132(10):1064-1074.
doi: 10.1182/blood-2017-12-820571 pmid: 29776906 |
| [10] |
Verrier S, Hogan A , McKie N, et al. ADAM gene expression and regulation during human osteoclast formation[J]. Bone, 2004,35(1):34-46.
doi: 10.1016/j.bone.2003.12.029 pmid: 15207739 |
| [11] |
Primakoff P, Myles DG . The ADAM gene family: surface proteins with adhesion and proteinase activity[J]. Trends Genets, 2000,16(2):83-87.
doi: 10.1016/S0168-9525(99)01926-5 |
| [12] |
Stone AT, Kroeger M, Amysang QX . Structure-function analysis of the adam family of disintegrin-like and metalloproteinase-containing p roteins[J]. J Protein Chem, 1999,18(4):447-465.
doi: 10.1023/A:1020692710029 |
| [13] |
Seals DF, Courtneidge SA . The ADAMs family of metalloprotea-ses: multidomain proteins with multiple functions[J]. Genes Dev, 2003,17(1):7-30.
doi: 10.1101/gad.1039703 pmid: 12514095 |
| [14] |
Hall KC, Hill D, Otero M , et al. ADAM17 controls endochondral ossification by regulating terminal differentiation of chondrocytes[J]. Mol Cell Biol, 2013,33(16):3077-3090.
doi: 10.1128/MCB.00291-13 pmid: 23732913 |
| [15] |
Saito K, Horiuchi K, Kimura T , et al. Conditional inactivation of TNFα-converting enzyme in chondrocytes results in an elongated growth plate and shorter long bones[J]. PLoS One, 2013,8(1):e54853.
doi: 10.1371/journal.pone.0054853 pmid: 3548805 |
| [16] |
Zunke F, Rose-John S . The shedding protease ADAM17: Physio-logy and pathophysiology[J]. Biochim Biophys Acta Mol Cell Res, 2017,1864(11 Pt B):2059-2070.
doi: 10.1016/j.bbamcr.2017.07.001 pmid: 28705384 |
| [17] |
Araya HF, Sepulveda H, Lizama CO , et al. Expression of the ectodomain-releasing protease ADAM17 is directly regulated by the osteosarcoma and bone-related transcription factor RUNX2[J]. J Cell Biochem, 2018,119(10):8204-8219.
doi: 10.1002/jcb.26832 |
| [18] |
Horiuchi K, Kimura T, Miyamoto T , et al. Conditional inactivation of TACE by a Sox9 promoter leads to osteoporosis and increased granulopoiesis via dysregulation of IL-17 and G-CSF[J]. J Immunol, 2009,182(4):2093-2101.
doi: 10.4049/jimmunol.0802491 pmid: 19201862 |
| [1] | 帅婷,刘娟,郭艳艳,金婵媛. 敲减长链非编码RNA MIR4697HG抑制骨髓间充质干细胞成脂向分化[J]. 北京大学学报(医学版), 2022, 54(2): 320-326. |
| [2] | 尤鹏越,刘玉华,王新知,王思雯,唐琳. 脱细胞猪心包膜生物相容性及成骨性能的体内外评价[J]. 北京大学学报(医学版), 2021, 53(4): 776-784. |
| [3] | 谢静,赵玉鸣,饶南荃,汪晓彤,方滕姣子,李晓霞,翟越,李静芝,葛立宏,王媛媛. 3种口腔颌面部来源的间充质干细胞成血管内皮分化潜能的比较研究[J]. 北京大学学报(医学版), 2019, 51(5): 900-906. |
| [4] | 朱云艳,李倩,张怡美,周彦恒. MAPK和AKT磷酸化下调参与Toll样受体抑制的人牙周膜干细胞的成骨分化[J]. 北京大学学报(医学版), 2018, 50(1): 33-41. |
|
||