1 资料与方法
1.1 一般资料
1.2 手术方法
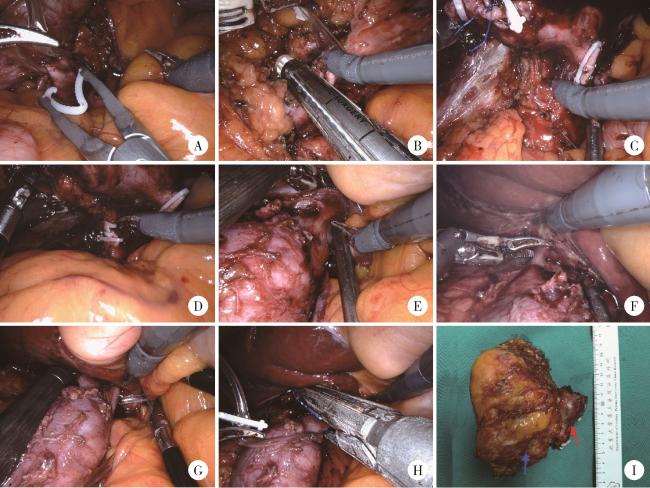
图1 右侧肾肿瘤患者行机器人辅助腹腔镜下腔静脉节段性切除术手术步骤Figure 1 Surgical procedures of robot-assisted laparoscopic segmental resection of inferior vena cava in the patient with right renal tumor A, clipping and transection of left renal vein; B, linear stapler division of distal inferior vena cava (IVC); C, dissection and exposure of posterior IVC plane; D, transection of right renal artery; E, isolation of short hepatic veins; F, Maryland forceps electrocautery division of second short hepatic vein; G, placement of vascular occlusion tape; H, proximal IVC transection with linear stapler; I, postoperative specimen (blue arrow indicating renal tumor, red arrow showing tumor thrombus). |
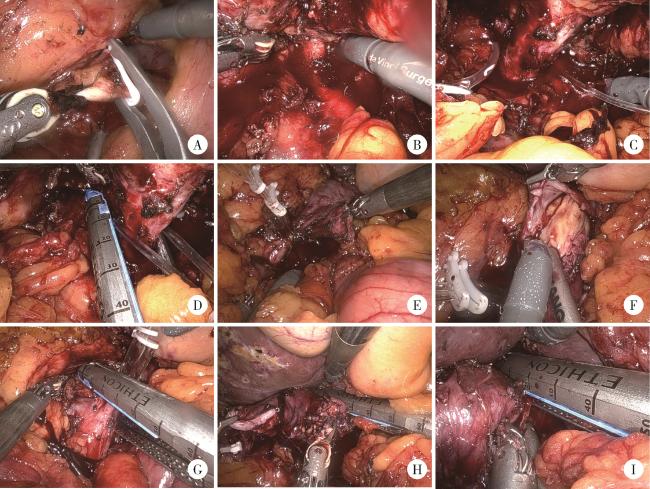
图2 左侧肾肿瘤患者行机器人辅助腹腔镜下腔静脉节段性切除术手术步骤Figure 2 Surgical procedures of robot-assisted laparoscopic segmental resection of inferior vena cava in the patient with left renal tumor A, division of left ureter; B, dissection and transection of left renal artery; C, dissection of left renal vein; D, transaction of left renal vein; E, exposure of inferior vena cava (IVC); F, tumor thrombus invades the wall of the vena cava; G, linear stapler division of distal IVC; H, proximal IVC transection with linear stapler; I, proximal IVC transection with linear stapler. |
1.3 术后随访
1.4 统计学分析
2 结果
表1 机器人辅助腹腔镜下腔静脉节段性切除患者的临床特征(n=44)Table 1 Patient characteristics of robot-assisted laparoscopic segmental resection of the inferior vena cava (n=44) |
| Items | Data |
| Age/years, median (P25, P75) | 62 (55, 68) |
| Male, n(%) | 31 (70.5) |
| Body mass index, median (P25, P75) | 24.5 (22.1, 27.3) |
| Side, n(%) | |
| Left | 5 (11.4) |
| Right | 39 (88.6) |
| Size/cm, median (P25, P75) | 8.1 (6.1, 10.1) |
| Mayo classification, n(%) | |
| Ⅰ | 0 (0) |
| Ⅱ | 37 (84.1) |
| Ⅲ | 6 (13.6) |
| Ⅳ | 1 (2.3) |
| ASA class, n(%) | |
| Ⅰ | 2 (4.5) |
| Ⅱ | 28 (63.6) |
| Ⅲ | 14 (31.8) |
| Operative time/min, median (P25, P75) | 224.0 (167.3, 303.8) |
| Estimated blood loss/mL, median (P25, P75) | 500.0 (300.0, 850.0) |
| Patients receiving transfusion, n(%) | 19 (43.2) |
| Lymph node dissection, n(%) | 29 (65.9) |
| Adrenalectomy, n(%) | 31 (70.5) |
| Postoperative hospital stay/d, median (P25, P75) | 6 (5, 8) |
| Preoperative serum creatinine/(μmol/L), median (P25, P75) | 102.5 (82.3, 115.3) |
| Postoperative serum creatinine/(μmol/L), median (P25, P75) | 116.0 (86.5, 157.5) |
| Venous tumor thrombus combined with bland thrombus, n(%) | 17 (38.6) |
| Histological type, n(%) | |
| Clear cell renal cell carcinoma | 34 (77.3) |
| Papillary renal cell carcinoma | 2 (4.5) |
| Unclassified renal cell carcinoma | 2 (4.5) |
| Spindle cell sarcoma | 1 (2.3) |
| Leiomyosarcomas | 1 (2.3) |
| TFE3-rearranged renal cell carcinoma | 1 (2.3) |
| Fumarate hydratase-deficient renal cell carcinoma | 1 (2.3) |
| Indeterminate histology due to treatment response | 2 (4.5) |
| Four-tiered WHO/ISUP grading system, n(%) | |
| Ⅰ | 1 (2.3) |
| Ⅱ | 8 (18.2) |
| Ⅲ | 10 (22.7) |
| Ⅳ | 15 (34.1) |
| Not graded | 10 (22.7) |
| T stage, n(%) | |
| T3b | 12 (27.3) |
| T3c | 29 (65.9) |
| T4 | 3 (6.8) |
| N1, n(%) | 8 (18.2) |
| M1, n(%) | 17 (38.6) |
| Adrenal metastasis, n(%) | 3 (6.8) |
| Invade the wall of the vena cava, n(%) | 36 (81.8) |
| Complication, n(%) | 25 (56.8) |
“Not graded” includes tumors with treatment-induced necrosis obscuring architecture, and subtypes without validated grading criteria. ASA, American Society of Anesthesiologists; WHO, World Health Organization; ISUP, International Society for Urological Pathology. |