Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (1): 188-194. doi: 10.19723/j.issn.1671-167X.2021.01.028
Previous Articles Next Articles
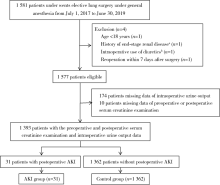
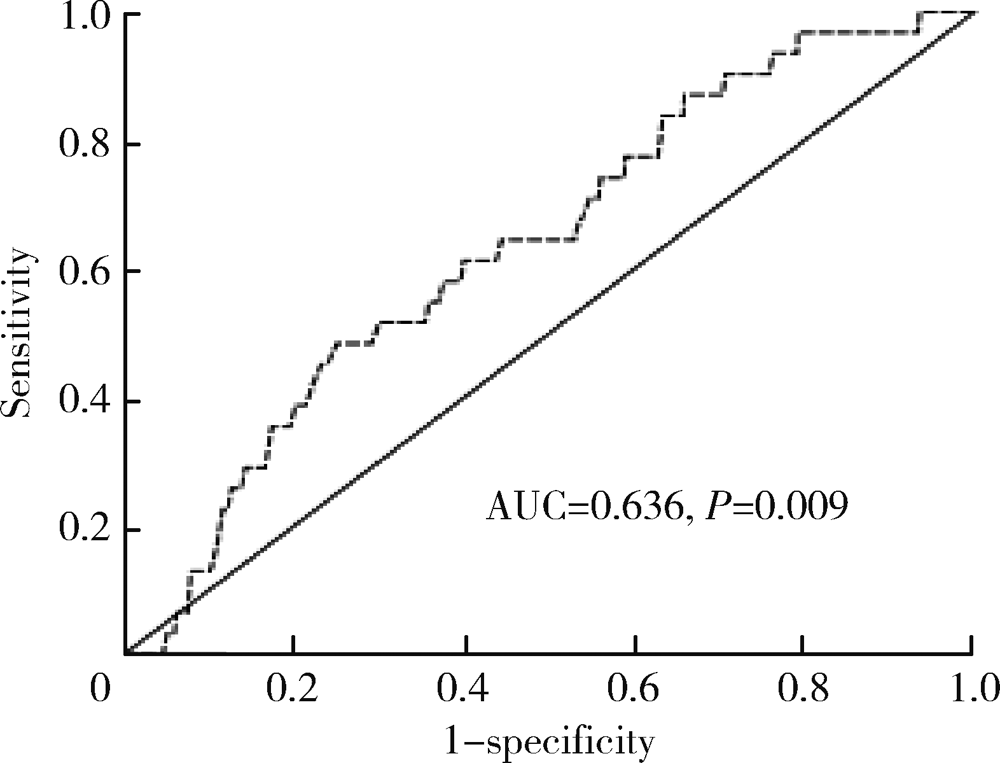
Impact of oliguria during lung surgery on postoperative acute kidney injury
MENG Zhao-ting,MU Dong-liang( )
)
- Department of Anesthesiology, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R614
| [1] | Nadim MK, Forni LG, Bihorac A, et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th International Consensus Conference of the ADQI (acute disease quality initiative) group[J]. J Am Heart Assoc, 2018,7(11):e8834. |
| [2] |
Sanaiha Y, Kavianpour B, Dobaria V, et al. Acute kidney injury is independently associated with mortality and resource use after emergency general surgery operations[J]. Surgery, 2020,167(2):328-334.
doi: 10.1016/j.surg.2019.07.035 pmid: 31668777 |
| [3] |
Vaara ST, Bellomo R. Postoperative renal dysfunction after noncardiac surgery[J]. Curr Opin Crit Care, 2017,23(5):440-446.
doi: 10.1097/MCC.0000000000000439 pmid: 28820797 |
| [4] |
Grams ME, Sang Y, Coresh J, et al. Acute kidney injury after major surgery: a retrospective analysis of veterans health administration data[J]. Am J Kidney Dis, 2016,67(6):872-880.
doi: 10.1053/j.ajkd.2015.07.022 pmid: 26337133 |
| [5] | Cardinale D, Cosentino N, Moltrasio M, et al. Acute kidney injury after lung cancer surgery: Incidence and clinical relevance, predictors, and role of N-terminal pro B-type natriuretic peptide[J]. Lung Cancer, 2018,123(9):155-159. |
| [6] |
Ostermann M, Joannidis M. Acute kidney injury 2016: diagnosis and diagnostic workup[J]. Crit Care, 2016,20(1):299-311.
doi: 10.1186/s13054-016-1478-z pmid: 27670788 |
| [7] |
Weiss R, Meersch M, Pavenstädt HJ, et al. Acute kidney injury: a frequently underestimated problem in perioperative medicine[J]. Dtsch Arztebl Int, 2019,116(49):833-842.
doi: 10.3238/arztebl.2019.0833 pmid: 31888797 |
| [8] |
Zarbock A, Koyner JL, Hoste EAJ, et al. Update on perioperative acute kidney injury[J]. Anesth Analg, 2018,127(5):1236-1245.
doi: 10.1213/ANE.0000000000003741 pmid: 30138176 |
| [9] |
du Toit L, Biccard BM. The relationship between intraoperative oliguria and acute kidney injury[J]. Br J Anaesth, 2019,122(6):707-710.
doi: 10.1016/j.bja.2019.03.008 pmid: 30961912 |
| [10] |
Hori D, Katz1 NM, Fine DM, et al. Defining oliguria during cardiopulmonary bypass and its relationship with cardiac surgery-associated acute kidney injury[J]. Br J Anaesth, 2016,117(6):733-740.
pmid: 27956671 |
| [11] |
Mizota T, Yamamoto Y, Hamada M, et al. Intraoperative oliguria predicts acute kidney injury after major abdominal surgery[J]. Br J Anaesth, 2017,119(6):1127-1134.
doi: 10.1093/bja/aex255 pmid: 29136086 |
| [12] |
Kim HJ, Cha SI, Kim CH, et al. Risk factors of postoperative acute lung injury following lobectomy for nonsmall cell lung cancer[J]. Medicine, 2019,98(13):e15078.
doi: 10.1097/MD.0000000000015078 pmid: 30921242 |
| [13] |
O’Connor ME, Kirwan CJ, Pearse RM, et al. Incidence and associations of acute kidney injury after major abdominal surgery[J]. Intensive Care Med, 2016,42(4):521-530.
doi: 10.1007/s00134-015-4157-7 pmid: 26602784 |
| [14] |
Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values[J]. Clin Chem, 2007,53(4):766-772.
doi: 10.1373/clinchem.2006.077180 pmid: 17332152 |
| [15] |
Quan S, Pannu N, Wilson T, et al. Prognostic implications of adding urine output to serum creatinine measurements for staging of acute kidney injury after majorsurgery: a cohort study[J]. Nephrol Dial Transplant, 2016,31(12):2049-2056.
doi: 10.1093/ndt/gfw374 pmid: 27941063 |
| [16] |
Kellum JA, Sileanu FE, Murugan R, et al. Classifying AKI by urine output versus serum creatinine level[J]. J Am Soc Nephrol, 2015,26(9):2231-2238.
doi: 10.1681/ASN.2014070724 pmid: 25568178 |
| [17] |
Eknoyan G. Rufus of ephesus and his “diseases of the kidneys”[J]. Nephron, 2002,91(3):383-390.
doi: 10.1159/000064277 pmid: 12119467 |
| [18] |
Macedo E, Malhotra R, Bouchard J, et al. Oliguria is an early predictor of higher mortality in critically ill patients[J]. Kidney Int, 2011,80(7):760-770.
doi: 10.1038/ki.2011.150 pmid: 21716258 |
| [19] | Inácio R, Gameiro J, Amaro S, et al. Intraoperative oliguria does not predict postoperative acute kidney injury in major abdominal surgery: a cohort analysis [J/OL]. J Bras Nefrol[2019-12-01]. https://doi.org/10.1590/2175-8239-jbn-2019-0244. |
| [20] |
Rung GW, Marshall WK. Nerve blocks in the critical care environment[J]. Crit Care Clin, 1990,6(2):343-367.
pmid: 2188709 |
| [21] |
Matot I, Dery E, Bulgov Y, et al. Fluid management during video-assisted thoracoscopic surgery for lung resection: a ran-domized, controlled trial of effects on urinary output and postoperative renal function[J]. J Thorac Cardiovasc Surg, 2013,146(2):461-466.
doi: 10.1016/j.jtcvs.2013.02.015 pmid: 23558303 |
| [22] |
Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus liberal fluid therapy for major abdominal surgery[J]. N Engl J Med, 2018,378(24):2263-2274.
doi: 10.1056/NEJMoa1801601 pmid: 29742967 |
| [1] | Zhicun LI, Tianyu WU, Lei LIANG, Yu FAN, Yisen MENG, Qian ZHANG. Risk factors analysis and nomogram model construction of postoperative pathological upgrade of prostate cancer patients with single core positive biopsy [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 896-901. |
| [2] | Ye YAN,Xiaolong LI,Haizhui XIA,Xuehua ZHU,Yuting ZHANG,Fan ZHANG,Ke LIU,Cheng LIU,Lulin MA. Analysis of risk factors for long-term overactive bladder after radical prostatectomy [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 589-593. |
| [3] | Yan CHEN,Kuangmeng LI,Kai HONG,Shudong ZHANG,Jianxing CHENG,Zhongjie ZHENG,Wenhao TANG,Lianming ZHAO,Haitao ZHANG,Hui JIANG,Haocheng LIN. Retrospective study on the impact of penile corpus cavernosum injection test on penile vascular function [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 680-686. |
| [4] | Bo PANG,Tongjun GUO,Xi CHEN,Huaqi GUO,Jiazhang SHI,Juan CHEN,Xinmei WANG,Yaoyan LI,Anqi SHAN,Hengyi YU,Jing HUANG,Naijun TANG,Yan WANG,Xinbiao GUO,Guoxing LI,Shaowei WU. Personal nitrogen oxides exposure levels and related influencing factors in adults over 35 years old in Tianjin and Shanghai [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 700-707. |
| [5] | Jing HE,Zhongze FANG,Ying YANG,Jing LIU,Wenyao MA,Yong HUO,Wei GAO,Yangfeng WU,Gaoqiang XIE. Relationship between lipid metabolism molecules in plasma and carotid atheroscle-rotic plaques, traditional cardiovascular risk factors, and dietary factors [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 722-728. |
| [6] | Shan CAI,Yihang ZHANG,Ziyue CHEN,Yunfe LIU,Jiajia DANG,Di SHI,Jiaxin LI,Tianyu HUANG,Jun MA,Yi SONG. Status and pathways of factors influencing physical activity time among elementary and junior high school students in Beijing [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 403-410. |
| [7] | Zuhong ZHANG,Tianjiao CHEN,Jun MA. Associations between puberty timing and cardiovascular metabolic risk factors among primary and secondary students [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 418-423. |
| [8] | Yuting LIN,Huali WANG,Yu TIAN,Litong GONG,Chun CHANG. Factors influencing cognitive function among the older adults in Beijing [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 456-461. |
| [9] | Jinrong ZHU,Yana ZHAO,Wei HUANG,Weiwei ZHAO,Yue WANG,Song WANG,Chunyan SU. Clinical characteristics of COVID-19 infection in patients undergoing hemodialysis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 267-272. |
| [10] | Zhanhong LAI,Jiachen LI,Zelin YUN,Yonggang ZHANG,Hao ZHANG,Xiaoyan XING,Miao SHAO,Yuebo JIN,Naidi WANG,Yimin LI,Yuhui LI,Zhanguo LI. A unicenter real-world study of the correlation factors for complete clinical response in idiopathic inflammatory myopathies [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 284-292. |
| [11] | Xiaoqian SI,Xiujuan ZHAO,Fengxue ZHU,Tianbing WANG. Risk factors for acute respiratory distress syndrome in patients with traumatic hemorrhagic shock [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 307-312. |
| [12] | Yangyang LI,Lin HOU,Zijun MA,Shanyamei HUANG,Jie LIU,Chaomei ZENG,Jiong QIN. Association of pregnancy factors with cow's milk protein allergy in infants [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 144-149. |
| [13] | Xiaoqiang LIU,Yin ZHOU. Risk factors of perioperative hypertension in dental implant surgeries with bone augmentation [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 93-98. |
| [14] | Liang LUO,Yun LI,Hong-yan WANG,Xiao-hong XIANG,Jing ZHAO,Feng SUN,Xiao-ying ZHANG,Ru-lin JIA,Chun LI. Anti-endothelial cell antibodies in predicting early miscarriage [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1039-1044. |
| [15] | Yu-fei LI,Ya-ni YAN,Jia-yang JIN,Chun LI,Qiu-yan PEI. Clinical characteristics of fetal cardiac disease in patients with anti-SSA antibody positive [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1053-1057. |
|
||