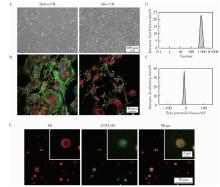
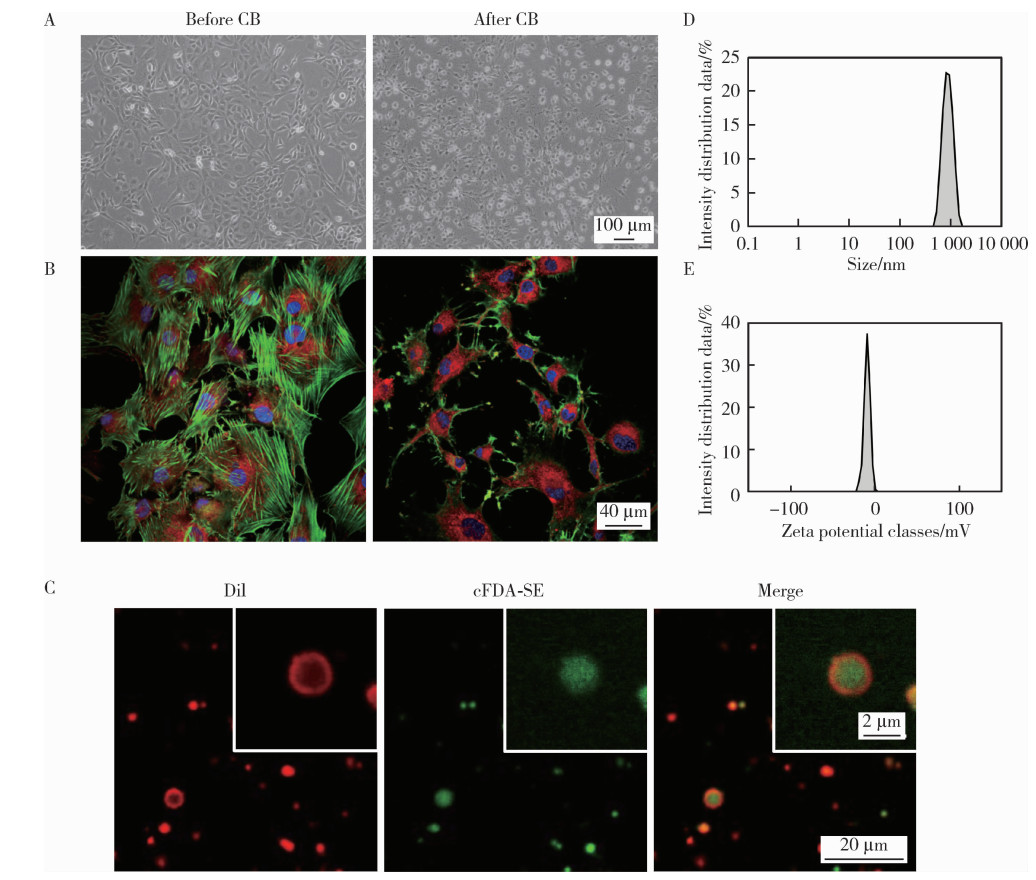
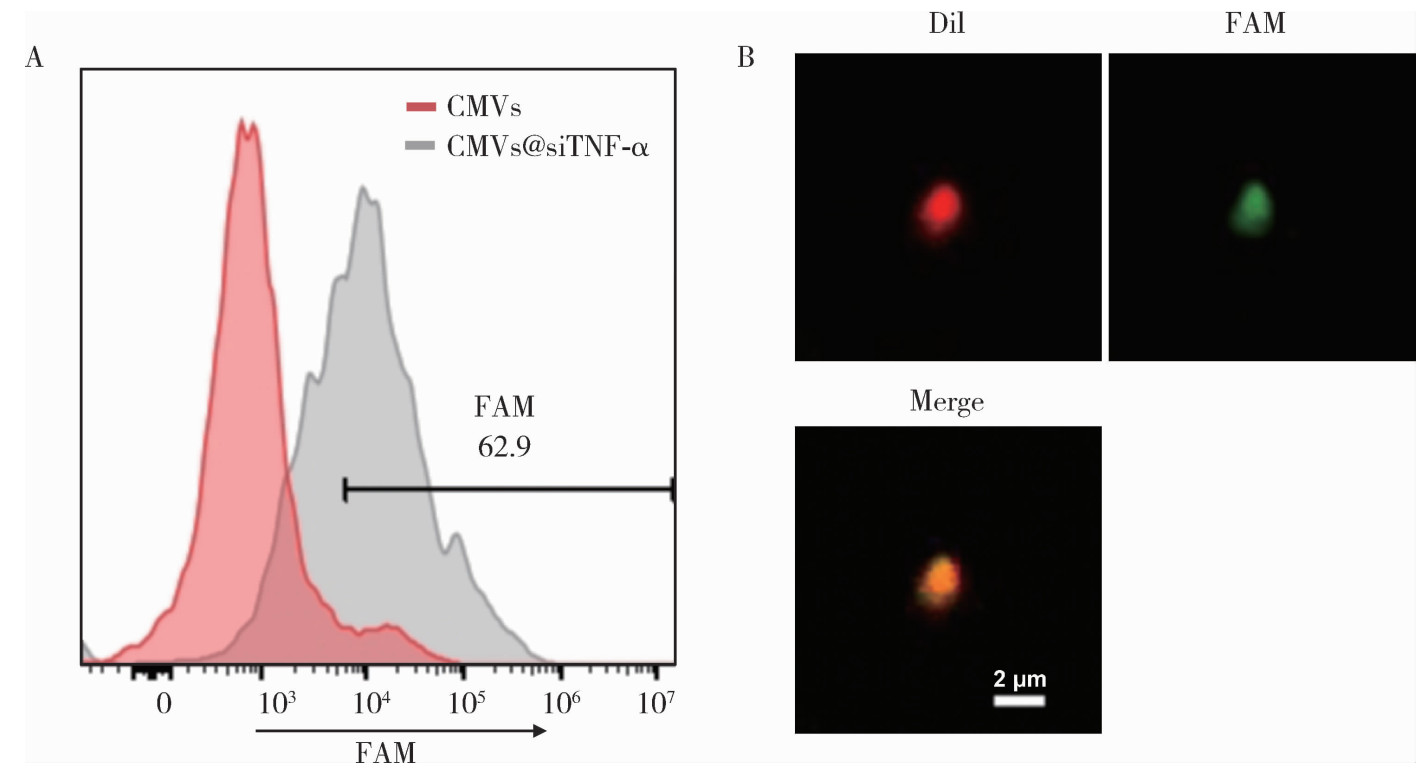
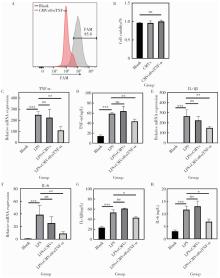
Journal of Peking University (Health Sciences) ›› 2026, Vol. 58 ›› Issue (1): 22-29. doi: 10.19723/j.issn.1671-167X.2026.01.003
Previous Articles Next Articles
Anti-inflammatory effects of cell membrane vesicle-mediated delivery of small interfering RNA targeting tumor necrosis factor-α on dental pulp stem cells
Ruofan GAO, Tianyu MA, Runkai WANG, Yuchen YIN, Ruidi LI, Dandan WANG*( ), Bin XIA*(
), Bin XIA*( )
)
- Department of Pediatric Dentistry, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing 100081, China
CLC Number:
- R78
| 1 |
doi: 10.1038/s41587-023-02105-y |
| 2 |
doi: 10.1016/j.ejphar.2021.174178 |
| 3 |
doi: 10.1016/j.ymthe.2024.01.005 |
| 4 |
doi: 10.1021/acs.accounts.9b00368 |
| 5 |
doi: 10.1038/s41573-024-00912-9 |
| 6 |
doi: 10.1093/nar/gky1239 |
| 7 |
doi: 10.1093/nar/gkx960 |
| 8 |
doi: 10.1093/nar/gkac539 |
| 9 |
doi: 10.1186/s12964-023-01103-6 |
| 10 |
doi: 10.1016/j.addr.2021.03.005 |
| 11 |
doi: 10.1002/advs.202101562 |
| 12 |
|
| 13 |
doi: 10.1016/j.jconrel.2018.02.031 |
| 14 |
doi: 10.3390/mi10110750 |
| 15 |
doi: 10.3390/ijms26094325 |
| 16 |
doi: 10.1111/iej.14108 |
| 17 |
doi: 10.1186/s12903-022-02161-x |
| 18 |
doi: 10.1177/154405910508401105 |
| 19 |
doi: 10.1186/s12974-025-03356-z |
| 20 |
doi: 10.3389/fcell.2025.1511577 |
| 21 |
doi: 10.1021/ja044605x |
| 22 |
doi: 10.1093/nar/gkaa670 |
| 23 |
doi: 10.1016/j.omtm.2025.101436 |
| 24 |
doi: 10.1021/acsami.5b05065 |
| 25 |
doi: 10.1042/BCJ20210584 |
| 26 |
doi: 10.1038/s41413-024-00325-9 |
| 27 |
doi: 10.1080/20013078.2017.1265291 |
| 28 |
doi: 10.1021/bc500291r |
| 29 |
doi: 10.1111/iej.14078 |
| 30 |
doi: 10.1186/s12974-023-02747-4 |
| 31 |
doi: 10.1136/ard-2022-222605 |
| 32 |
doi: 10.1038/s41577-024-01008-6 |
| 33 |
doi: 10.1021/acsnano.1c03800 |
| [1] | Mengdi LI, Lei LEI, Zhongning LIU, Jian LI, Ting JIANG. Gene silencing of Nemo-like kinase promotes neuralized tissue engineered bone regeneration [J]. Journal of Peking University (Health Sciences), 2025, 57(2): 227-236. |
| [2] | Yu-yang YE,Lin YUE,Xiao-ying ZOU,Xiao-yan WANG. Characteristics and microRNA expression profile of exosomes derived from odontogenic dental pulp stem cells [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 689-696. |
| [3] | LOU Xue,LIAO Li,LI Xing-jun,WANG Nan,LIU Shuang,CUI Ruo-mei,XU Jian. Methylation status and expression of TWEAK gene promoter region in peripheral blood of patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1020-1025. |
| [4] | Yong-wei HU,Rui LIU,Li LUO. Chronic multifocal osteomyelitis: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1140-1145. |
| [5] | Xiao-min GAO,Xiao-ying ZOU,Lin YUE. Mediated pathways of exosomes uptake by stem cells of apical papilla [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 43-50. |
| [6] | Jing XIE,Yu-ming ZHAO,Nan-quan RAO,Xiao-tong WANG,Teng-jiao-zi FANG,Xiao-xia LI,Yue ZHAI,Jing-zhi LI,Li-hong GE,Yuan-yuan WANG. Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 900-906. |
| [7] | JIA Wei-qian, ZHAO Yu-ming, GE Li-hong. Recombinant human transforming growth factor β1 promotes dental pulp stem cells proliferation and mineralization [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 680-681. |
|
||