Journal of Peking University(Health Sciences) ›› 2018, Vol. 50 ›› Issue (6): 1027-1032. doi: 10.19723/j.issn.1671-167X.2018.06.015
• Article • Previous Articles Next Articles
Increased serum soluble-endoglin level and its clinical significance in antiphospholipid syndrome
Ji LI1,2,Li ZHENG1,Lian-jie SHI2,Jing XU2,Jian-long SHU3,Xue-wu ZHANG3,△( )
)
- 1. Department of Rheumatology and Immunology, Peking University People’ s Hospital, Beijing 100044, China
2. Department of Rheumatology and Immunology, Peking University International Hospital, Beijing 102206, China
3. Department of Rheumatology and Immunology, Guangxi International Zhuang Medical Hospital, Nanning 530011, China
CLC Number:
- R593.2
| [1] |
Miyakis S, Lockshin MD, Atsumi T , et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS)[J]. J Thromb Haemost, 2006,4(2):295-306.
doi: 10.1111/jth.2006.4.issue-2 |
| [2] |
Pierangeli SS, Colden-Stanfield M, Liu X , et al. Antiphospholi-pid antibodies from antiphospholipid syndrome patients activate endothelial cells in vitro and in vivo[J]. Circulation, 1999,99(15):1997-2002.
doi: 10.1161/01.CIR.99.15.1997 |
| [3] |
Dunoyer-Geindre S, de Moerloose P, Galve-de Rochemonteix B , et al. NFκB is an essential intermediate in the activation of endothelial cells by anti-β2-glycoprotein 1 antibodies[J]. Thromb Haemost, 2002,88(5):851-857.
doi: 10.1055/s-0037-1613313 |
| [4] |
Charakida M, Besler C, Batuca JR , et al. Vascular abnormalities, paraoxonase activity, and dysfunctional HDL in primary antiphospholipid syndrome[J]. JAMA, 2009,302(11):1210-1217.
doi: 10.1001/jama.2009.1346 |
| [5] |
Ames PR, Batuca JR, Ciampa A , et al. Clinical relevance of nitric oxide metabolites and nitrative stress in thrombotic primary antiphospholipid syndrome[J]. J Rheumatol, 2010,37(12):2523-2530.
doi: 10.3899/jrheum.100494 pmid: 20889602 |
| [6] |
Ramesh S, Morrell CN, Tarango C , et al. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via β2GPI and apoER2[J]. J Clin Invest, 2011,121(1):120-131.
doi: 10.1172/JCI39828 pmid: 3007129 |
| [7] |
Toporsian M, Gros R, Kabir MG , et al. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia[J]. Circ Res, 2005,96(6):684-692.
doi: 10.1161/01.RES.0000159936.38601.22 pmid: 15718503 |
| [8] |
Alt A, Miguel-Romero L, Donderis J , et al. Structural and functional insights into endoglin ligand recognition and binding[J]. PLoS One, 2012,7(2):e29948.
doi: 10.1371/journal.pone.0029948 pmid: 22347366 |
| [9] |
Bernabeu C, Conley BA, Vary CP . Novel biochemical pathways of endoglin in vascular cell physiology[J]. J Cell Biochem, 2007,102(6):1375-1388.
doi: 10.1002/jcb.21594 pmid: 17975795 |
| [10] |
Blazquez-Medela AM, Garcia-Ortiz L, Gomez-Marcos MA , et al. Increased plasma soluble endoglin levels as an indicator of cardiovascular alterations in hypertensive and diabetic patients[J]. BMC Med, 2010,8:86.
doi: 10.1186/1741-7015-8-86 pmid: 21171985 |
| [11] |
Strasky Z, Vecerova L, Rathouska J , et al. Cholesterol effects on endoglin and its downstream pathways in ApoE/LDLR double knockout mice[J]. Circ J, 2011,75(7):1747-1755.
doi: 10.1253/circj.CJ-10-1285 pmid: 21576826 |
| [12] |
Vitverova B, Blazickova K, Najmanova I , et al. Soluble endoglin and hypercholesterolemia aggravate endothelial and vessel wall dysfunction in mouse aorta[J]. Atherosclerosis, 2018,271:15-25.
doi: 10.1016/j.atherosclerosis.2018.02.008 pmid: 29459262 |
| [13] |
Emeksiz HC, Bideci A, Damar Ç , et al. Soluble endoglin level increase occurs prior to development of subclinical structural vascular alterations in diabetic adolescents[J]. J Clin Res Pediatr Endocrinol, 2016,8(3):313-320.
doi: 10.4274/jcrpe |
| [14] |
Rathouska J, Jezkova K, Nemeckova I , et al. Soluble endoglin, hypercholesterolemia and endothelial dysfunction[J]. Atherosclerosis, 2015,243(2):383-388.
doi: 10.1016/j.atherosclerosis.2015.10.003 pmid: 26520890 |
| [15] |
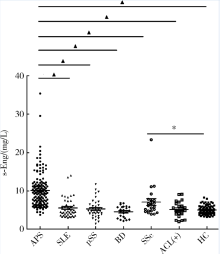
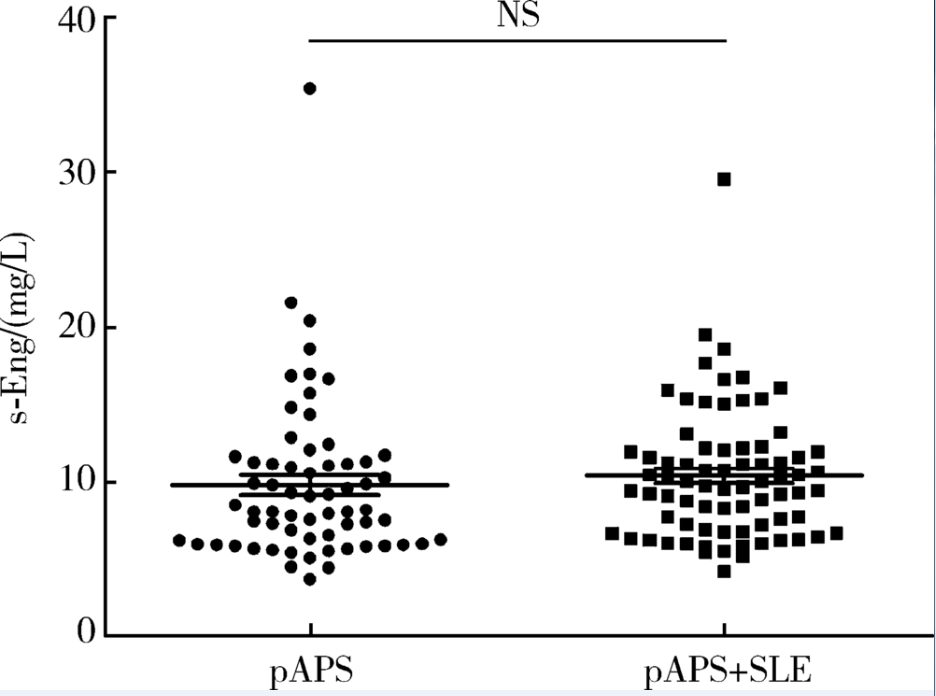
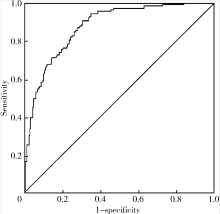
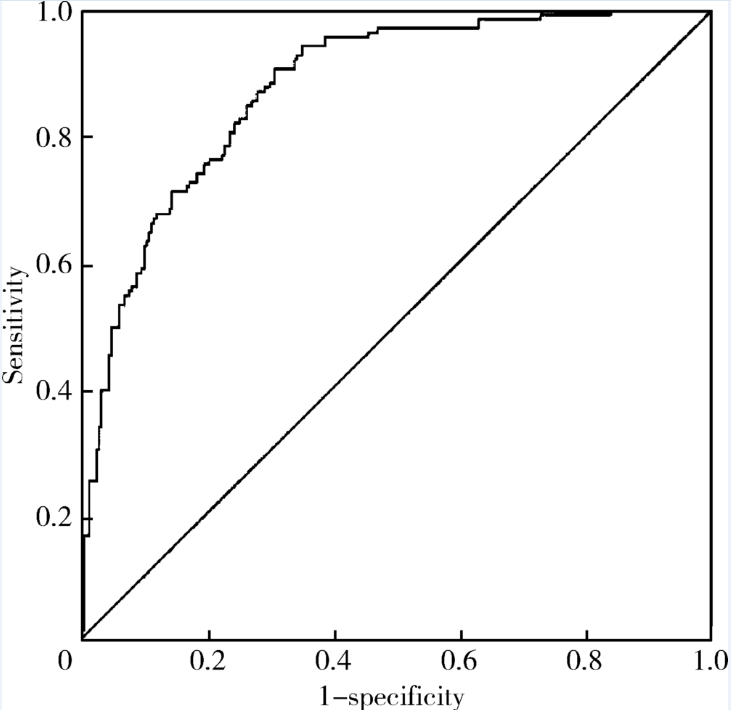
Bassyouni IH, El-Shazly R, Azkalany GS , et al. Clinical significance of soluble-endoglin levels in systemic lupus erythematosus: possible association with anti-phospholipid syndrome[J]. Lupus, 2012,21(14):1565-1570.
doi: 10.1177/0961203312460115 pmid: 22941564 |
| [16] |
Barbara NP, Wrana JL, Letarte M . Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily[J]. J Biol Chem, 1999,274(2):584-594.
doi: 10.1074/jbc.274.2.584 |
| [17] |
Kapur NK, Wilson S, Yunis AA , et al. Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure[J]. Circulation, 2012,125(22):2728-2738.
doi: 10.1161/CIRCULATIONAHA.111.080002 |
| [18] |
Venkatesha S, Toporsian M, Lam C , et al. Soluble endoglin contributes to the pathogenesis of preeclampsia[J]. Nat Med, 2006,12(6):642-649.
doi: 10.1038/nm1429 pmid: 16751767 |
| [19] |
Chatterjee A, Black SM, Catravas JD . Endothelial nitric oxide (NO) and its pathophysiologic regulation[J]. Vascul Pharmacol, 2008,49(4/5/6):134-140.
doi: 10.1016/j.vph.2008.06.008 pmid: 2592563 |
| [20] |
Palmer RM, Ferrige AG, Moncada S . Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor[J]. Nature, 1987,327(6122):524-526.
doi: 10.1038/327524a0 |
| [21] |
de Caterina R, Libby P, Peng HB , et al. Nitric oxide decreases cytokine-induced endothelial activation: nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines[J]. J Clin Invest, 1995,96(1):60-68.
doi: 10.1172/JCI118074 |
| [22] |
Radomski MW, Palmer RM, Moncada S . Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium[J]. Lancet, 1987,2(8567):1057-1058.
doi: 10.1016/S0140-6736(87)91481-4 pmid: 2889967 |
| [23] |
Lauer T, Preik M, Rassaf T , et al. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action[J]. Proc Natl Acad Sci USA, 2001,98(22):12814-12819.
doi: 10.1073/pnas.221381098 pmid: 11606734 |
| [24] |
Robak E, Kierstan M, Cebula B , et al. Circulating endothelial cells and angiogenic proteins in patients with systemic lupus erythematosus[J]. Lupus, 2009,18(4):332-341.
doi: 10.1177/0961203308097572 pmid: 19276301 |
| [25] |
Cervera R, Serrano R, Pons-Estel GJ , et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients[J]. Ann Rheum Dis, 2015,74(6):1011-1018.
doi: 10.1136/annrheumdis-2013-204838 pmid: 24464962 |
| [26] |
Derksen RH, de Groot PG . The obstetric antiphospholipid syndrome[J]. J Reprod Immunol, 2008,77(1):41-50.
doi: 10.1016/j.jri.2006.12.003 |
| [27] |
Holers VM, Girardi G, Mo L , et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss[J]. J Exp Med, 2002,195(2):211-220.
doi: 10.1084/jem.200116116 pmid: 11805148 |
| [28] |
Soh MC, Pasupathy D, Gray G , et al. Persistent antiphospholipid antibodies do not contribute to adverse pregnancy outcomes[J]. Rheumatology(Oxford), 2013,52(9):1642-1647.
doi: 10.1093/rheumatology/ket173 pmid: 3741478 |
| [29] |
Levine RJ, Lam C, Qian C , et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia[J]. N Eng J Med, 2006,355(10):992-1005.
doi: 10.1056/NEJMoa055352 pmid: 15805796 |
| [1] | Xiaofei TANG,Yonghong LI,Qiuling DING,Zhuo SUN,Yang ZHANG,Yumei WANG,Meiyi TIAN,Jian LIU. Incidence and risk factors of deep vein thrombosis in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 279-283. |
| [2] | Li-rong HONG,Yu-jia CHEN,Qing-lai JIANG,Ru-lin JIA,Chun LI,Liang-hua FENG. Predictive value of four items of new thrombus markers combined with conventional coagulation tests for thrombosis in antiphospholipid syndrome [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1033-1038. |
| [3] | Jin-man ZHUANG,Tian-run LI,Xuan LI,Jing-yuan LUAN,Chang-ming WANG,Qi-chen FENG,Jin-tao HAN. Application of Rotarex catheter system in femoropopliteal artery stenosis accompanied with thrombosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 328-332. |
| [4] | Yu-ke HOU,Qing-meng CAI,Xiang-jun LIU,Ze-lin YUN,Chun LI,Xue-wu ZHANG. Clinical significance of oxidized low-density lipoprotein antibody in antiphospholipid syndrome [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1117-1122. |
| [5] | Rui LIU,Jin-xia ZHAO,Liang YAN. Clinical characteristics of patients with rheumatoid arthritis complicated with venous thrombosis of lower extremities [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1079-1085. |
| [6] | Yu-hua WANG,Guo-hua ZHANG,Ling-ling ZHANG,Jun-li LUO,Lan GAO. Adrenal hemorrhage in a patient with systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1178-1181. |
| [7] | Jie-yu GU,Cui LU,Hui SHI,Cheng-de YANG. Case series and clinical analysis of 14 cases of catastrophic antiphospholipid syndrome [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1033-1038. |
| [8] | ZHENG Xiao-Juan, DENG Xiao-Li, LIU Xiang-Yuan. Pregnancy outcome in 54 patients with antiphospholipid syndrome:a retrospective clinical study [J]. Journal of Peking University(Health Sciences), 2014, 46(2): 323-328. |
|
||