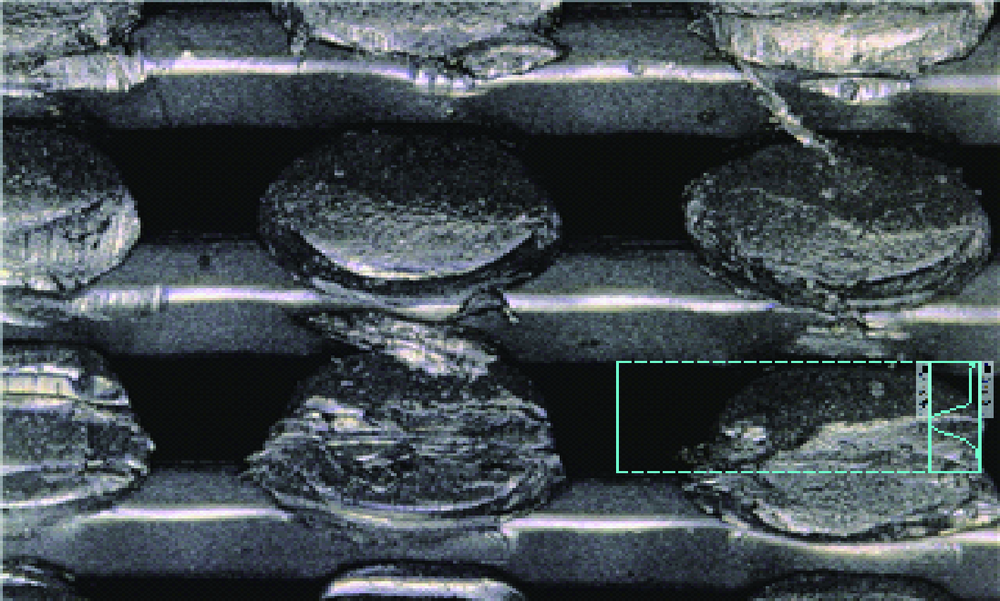
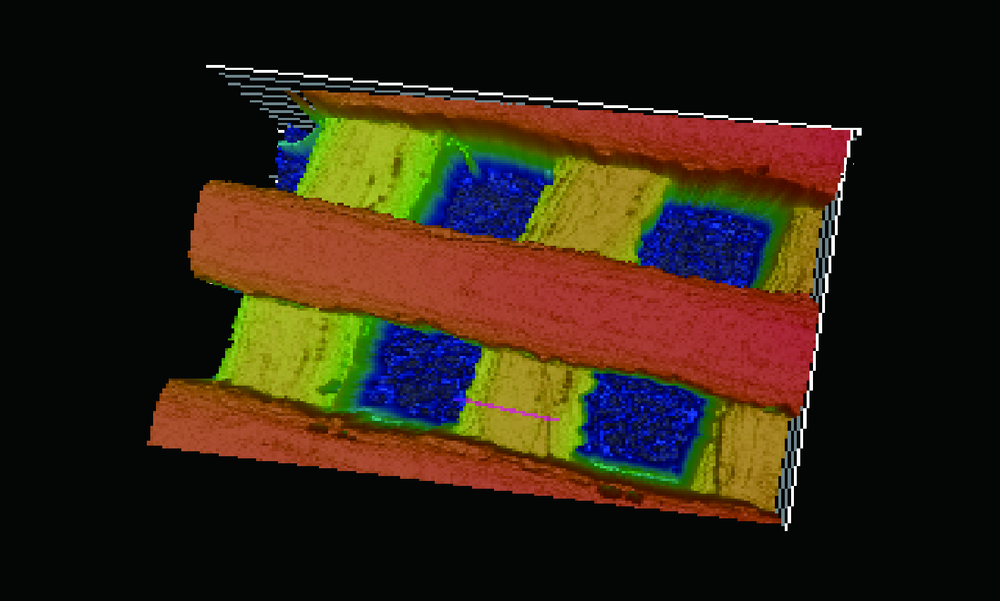
Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (1): 115-119. doi: 10.19723/j.issn.1671-167X.2019.01.021
Previous Articles Next Articles
Establishment of a 3D printing system for bone tissue engineering scaffold fabrication and the evaluation of its controllability over macro and micro structure precision
Rong LI1,Ke-long CHEN2,Yong WANG1,Yun-song LIU1,Yong-sheng ZHOU1,△( ),Yu-chun SUN1,△(
),Yu-chun SUN1,△( )
)
- 1. Center for Digital Dentistry, Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. Shinotech Co., Ltd, Beijing 100080, China
CLC Number:
- R78
| [1] |
Langer R, Vacanti JP . Tissue engineering[J]. Science, 1993,260(5110):920-926.
doi: 10.1126/science.8493529 |
| [2] |
Kneser U, Schaefer DJ, Polykandriotis E , et al. Tissue engineering of bone: the reconstructive surgeon’s point of view[J]. J Cell Mol Med, 2006,10(1):7-19.
doi: 10.1111/jcmm.2006.10.issue-1 |
| [3] |
Hutmacher DW . Scaffolds in tissue engineering bone and cartilage[J]. Biomaterials, 2000,21(24):2529-2543.
doi: 10.1016/S0142-9612(00)00121-6 pmid: 11071603 |
| [4] |
Jia A, Joanne EM, Ratima S , et al. Design and 3D printing of scaffolds and tissues[J]. Engineering, 2015,1(2):261-268.
doi: 10.15302/J-ENG-2015061 |
| [5] |
Peltola SM, Melchels FP, Grijpma DW , et al. A review of rapid prototyping techniques for tissue engineering purposes[J]. Ann Med, 2008,40(4):268-280.
doi: 10.1080/07853890701881788 |
| [6] |
Rezwan K, Chen QZ, Blaker JJ , et al. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering[J]. Biomaterials, 2006,27(18):3413-3431.
doi: 10.1016/j.biomaterials.2006.01.039 pmid: 16504284 |
| [7] |
Hulbert SF, Young FA, Mathews RS , et al. Potential of ceramic materials as permanently implantable skeletal prostheses[J]. J Biomed Mater Res, 1970,4(3):433-456.
doi: 10.1002/jbm.820040309 pmid: 5469185 |
| [8] |
Kuboki Y, Jin Q, Takita H . Geometry of carriers controlling phenotypic expression in BMP-induced osteogenesis and chondrogenesis[J]. J Bone Joint Surg Am, 2001,83(A Suppl 1Pt 2):S105-115.
doi: 10.1054/arth.2001.9052 pmid: 11314788 |
| [9] |
Tarawneh AM, Wettergreen M, Liebschner MAK . Computer-aided tissue engineering: benefiting from the control over scaffold micro-architecture[J]. Methods Mol Biol, 2012,868:1-25.
doi: 10.1007/978-1-61779-764-4 |
| [10] |
Campos Marin A, Lacroix D . The inter-sample structural variability of regular tissue-engineered scaffolds significantly affects the micromechanical local cell environment[J]. Interface Focus, 2015,5(2):20140097.
doi: 10.1098/rsfs.2014.0097 pmid: 4342953 |
| [11] |
Yu W, Hong Q, Hu G , et al. A Microfluidic-based multi-shear device for investigating the effects of low fluid-induced stresses on osteoblasts[J]. PLoS One, 2014,9(2):e89966.
doi: 10.1371/journal.pone.0089966 pmid: 24587156 |
| [12] |
Tarafder S, Balla VK, Davies NM , et al. Microwave sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering[J]. J Tissue Eng Regen Med, 2013,7(8):631-641.
doi: 10.1002/term.555 pmid: 22396130 |
| [13] |
李树袆, 周苗, 赖毓霄 , 等. 三维打印聚乳酸-羟基乙酸/磷酸三钙骨修复支架的生物学评价[J]. 中华口腔医学杂志, 2016,51(11):661-666.
doi: 10.3760/cma.j.issn.1002-0098.2016.11.005 |
| [14] |
Bergsma JE, Bruijn WCD, Rozema FR , et al. Late degradation tissue response to poly(l-lactide) bone plates and screws[J]. Biomaterials, 1995,16(1):25-31.
doi: 10.1016/0142-9612(95)91092-D pmid: 7718688 |
| [15] |
Böstman O, Hirvensalo E, Mäkinen J , et al. Foreign-body reactions to fracture fixation implants of biodegradable synthetic polymers[J]. J Bone Joint Surg Br, 1990,72(4):592-596.
doi: 10.1016/0020-1383(90)90021-L pmid: 2199452 |
| [16] |
Woodruff MA, Hutmacher DW . The return of a forgotten polymer & mdash; polycaprolactone in the 21st century[J]. Prog Polym Sci, 2010,35(10):1217-1256.
doi: 10.1016/j.progpolymsci.2010.04.002 |
| [17] |
Xu N, Ye X, Wei D , et al. 3D artificial bones for bone repair prepared by computed tomography-guided fused deposition modeling for bone repair[J]. ACS Appl Mater Interfaces, 2014,6(17):14952-14963.
doi: 10.1021/am502716t pmid: 25133309 |
| [18] |
Li Y, Wu ZG, Li XK , et al. A polycaprolactone-tricalcium phosphate composite scaffold as an autograft-free spinal fusion cage in a sheep model[J]. Biomaterials, 2014,35(22):5647-5659.
doi: 10.1016/j.biomaterials.2014.03.075 pmid: 24743032 |
| [1] | Yu-ke LI,Mei WANG,Lin TANG,Yu-hua LIU,Xiao-ying CHEN. Effect of pH on the chelation between strontium ions and decellularized small intestinal submucosal sponge scaffolds [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 44-51. |
| [2] | ABUDUREHEMAN Kaidierya,Rong-geng ZHANG,Hao-nan QIAN,Zhen-yang ZOU,YESITAO Danniya,Tian-yuan FAN. Preparation and in vitro evaluation of FDM 3D printed theophylline tablets with personalized dosage [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1202-1207. |
| [3] | Zhi-sheng LI,Hao-nan QIAN,Tian-yuan FAN. Preparation and in vitro evaluation of fused deposition modeling 3D printed compound tablets of captopril and hydrochlorothiazide [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 572-577. |
| [4] | Yi DENG,Yi ZHANG,Bo-wen LI,Mei WANG,Lin TANG,Yu-hua LIU. Effects of different crosslinking treatments on the properties of decellularized small intestinal submucosa porous scaffolds [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 557-564. |
| [5] | CHEN Di,XU Xiang-yu,WANG Ming-rui,LI Rui,ZANG Gen-ao,ZHANG Yue,QIAN Hao-nan,YAN Guang-rong,FAN Tian-yuan. Preparation and in vitro evaluation of fused deposition modeling 3D printed verapa-mil hydrochloride gastric floating formulations [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 348-354. |
| [6] | Mei WANG, Bo-wen LI, Si-wen WANG, Yu-hua LIU. Preparation and osteogenic effect study of small intestinal submucosa sponge [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 952-958. |
| [7] | Chun-ling CAO,Cong-chong YANG,Xiao-zhong QU,Bing HAN,Xiao-yan WANG. Effects of the injectable glycol-chitosan based hydrogel on the proliferation and differentiation of human dental pulp cells [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 10-17. |
| [8] | Qian-li ZHANG,Chong-yang YUAN,Li LIU,Shi-peng WEN,Xiao-yan WANG. Effects of electrospun collagen nanofibrous matrix on the biological behavior of human dental pulp cells [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 28-34. |
| [9] | CHEN Hu, ZHAO Tian, WANG Yong, SUN Yu-chun. Computer aided design and 3-dimensional printing for the production of custom trays of maxillary edentulous jaws based on 3-dimensional scan of primary impression [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 900-904. |
| [10] | LIU Yan, FU Yu, LIU Shuai, ZHOU Yan-heng. Effects of microstructure of mineralized collagen scaffolds on cell morphology of MG 63 [J]. Journal of Peking University(Health Sciences), 2014, 46(1): 19-24. |
|
||