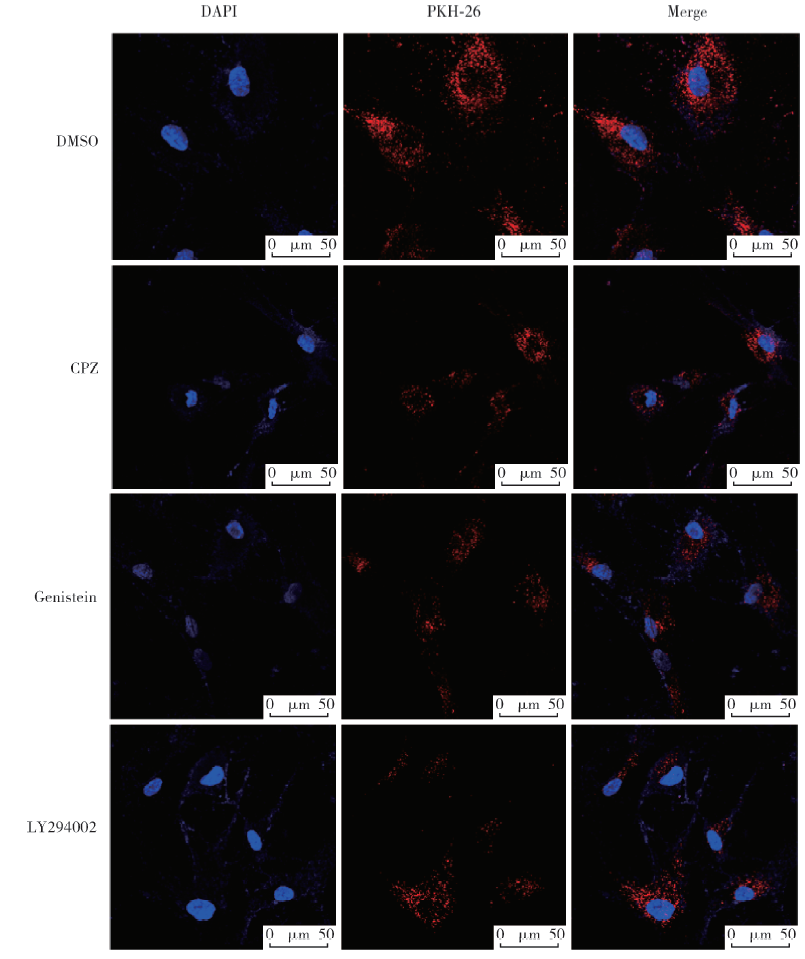
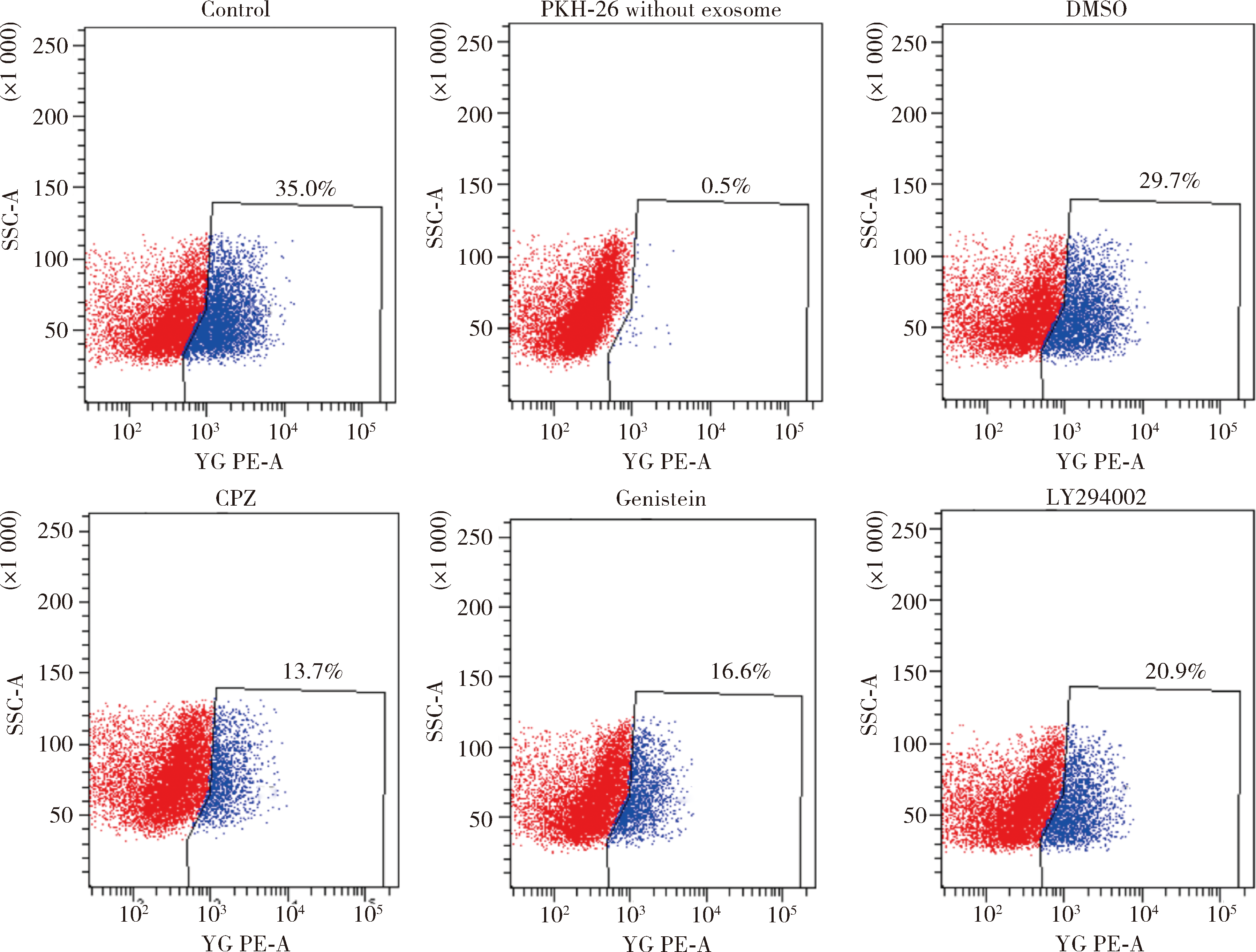
Journal of Peking University(Health Sciences) ›› 2020, Vol. 52 ›› Issue (1): 43-50. doi: 10.19723/j.issn.1671-167X.2020.01.007
Previous Articles Next Articles
Mediated pathways of exosomes uptake by stem cells of apical papilla
Xiao-min GAO,Xiao-ying ZOU( ),Lin YUE(
),Lin YUE( )
)
- Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology & National Clinical research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R329.2
| [1] | Vlassov AV, Magdaleno S, Setterquist R , et al. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials[J]. Biochim Biophys Acta, 2012,1820(7):940-948. |
| [2] | Johnstone RM, Mathew A, Mason AB , et al. Exosome formation during maturation of mammalian and avian reticulocytes: Evidence that exosome release is a major route for externalization of obsolete membrane proteins[J]. J Cell Physiol, 1991,147(1):27-36. |
| [3] | Raposo G, Nijman HW, Stoorvogel W , et al. B lymphocytes secrete antigen-presenting vesicles[J]. J Exp Med, 1996,183(3):1161-1172. |
| [4] | Valadi H, Ekstrom K, Bossios A , et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells[J]. Nat Cell Biol, 2007,9(6):654-672. |
| [5] | Huang CC, Narayanan R, Alapati S , et al. Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration[J]. Biomaterials, 2016,111:103-115. |
| [6] | Xian XH, Gong QM, Li C , et al. Exosomes with highly angioge-nic potential for possible use in pulp regeneration[J]. J Endod, 2018,44(5):751-758. |
| [7] | Liu JY, Chen X, Yue L , et al. CXC chemokine receptor 4 is expressed paravascularly in apical papilla and coordinates with stromal cell-derived factor-1α during transmigration of stem cells from apical papilla[J]. J Endod, 2015,41(9):1430-1436. |
| [8] | 刘敬一, 邹晓英, 陈雪 , 等. 脂多糖对人根尖牙乳头干细胞中基质细胞衍生因子1表达的影响[J]. 中华口腔医学杂志, 2015,50(6):346-351. |
| [9] | Lotvall J, Hill AF, Hochberg F , et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles[J]. J Extracell Vesicles, 2014,3:26913. |
| [10] | Pivoraite U, Jarmalaviciute A, Tunaitis V , et al. Exosomes from human dental pulp stem cells suppress carrageenan-induced acute inflammation in mice[J]. Inflammation, 2015,38(5):1933-1941. |
| [11] | Sokolova V, Ludwig AK, Hornung S , et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy[J]. Colloids Surf B Biointerfaces, 2011,87(1):146-150. |
| [12] | Tian T, Zhu YL, Hu FH , et al. Dynamics of exosome internalization and trafficking[J]. J Cell Physiol, 2013,228(7):1487-1495. |
| [13] | He ZL, Liu KZ, Manaloto E , et al. Cold atmospheric plasma induces ATP-dependent endocytosis of nanoparticles and synergistic U373MG cancer cell death[J]. Sci Rep, 2018,8(1):5298. |
| [14] | Kusuma RJ, Manca S, Friemel T , et al. Human vascular endothelial cells transport foreign exosomes from cow’s milk by endocytosis[J]. Am J Physiol Cell Physiol, 2016,310(10):C800-C807. |
| [15] | Tian T, Zhu YL, Zhou YY , et al. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery[J]. J Biol Chem, 2014,289(32):22258-22267. |
| [16] | Horibe S, Tanahashi T, Kawauchi S , et al. Mechanism of reci-pient cell-dependent differences in exosome uptake[J]. Bmc Can-cer, 2018,18(1):47. |
| [1] | Wen-gen LI,Xiao-dong GU,Rui-qiang WENG,Su-dong LIU,Chao CHEN. Expression and clinical significance of plasma exosomal miR-34-5p and miR-142-3p in systemic sclerosis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1022-1027. |
| [2] | Yu-yang YE,Lin YUE,Xiao-ying ZOU,Xiao-yan WANG. Characteristics and microRNA expression profile of exosomes derived from odontogenic dental pulp stem cells [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 689-696. |
| [3] | PANG Yong,ZHANG Sha,YANG Hua,ZHOU Rou-li. Serum LAPTM4B-35 protein as a novel diagnostic marker for hepatocellular carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 710-715. |
| [4] | Jing XIE,Yu-ming ZHAO,Nan-quan RAO,Xiao-tong WANG,Teng-jiao-zi FANG,Xiao-xia LI,Yue ZHAI,Jing-zhi LI,Li-hong GE,Yuan-yuan WANG. Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 900-906. |
| [5] | Xin-yun YAO,Xiao-min GAO,Xiao-ying ZOU,Lin YUE. Role of endocytosis in cell surface CXC chemokine receptor 4 expression of stem cells from apical papilla [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 893-899. |
| [6] | JIA Wei-qian, ZHAO Yu-ming, GE Li-hong. Recombinant human transforming growth factor β1 promotes dental pulp stem cells proliferation and mineralization [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 680-681. |
|
||