Journal of Peking University(Health Sciences) ›› 2020, Vol. 52 ›› Issue (1): 35-42. doi: 10.19723/j.issn.1671-167X.2020.01.006
Previous Articles Next Articles
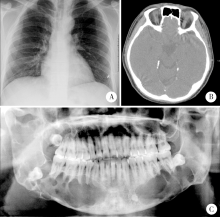
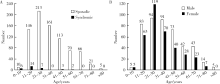
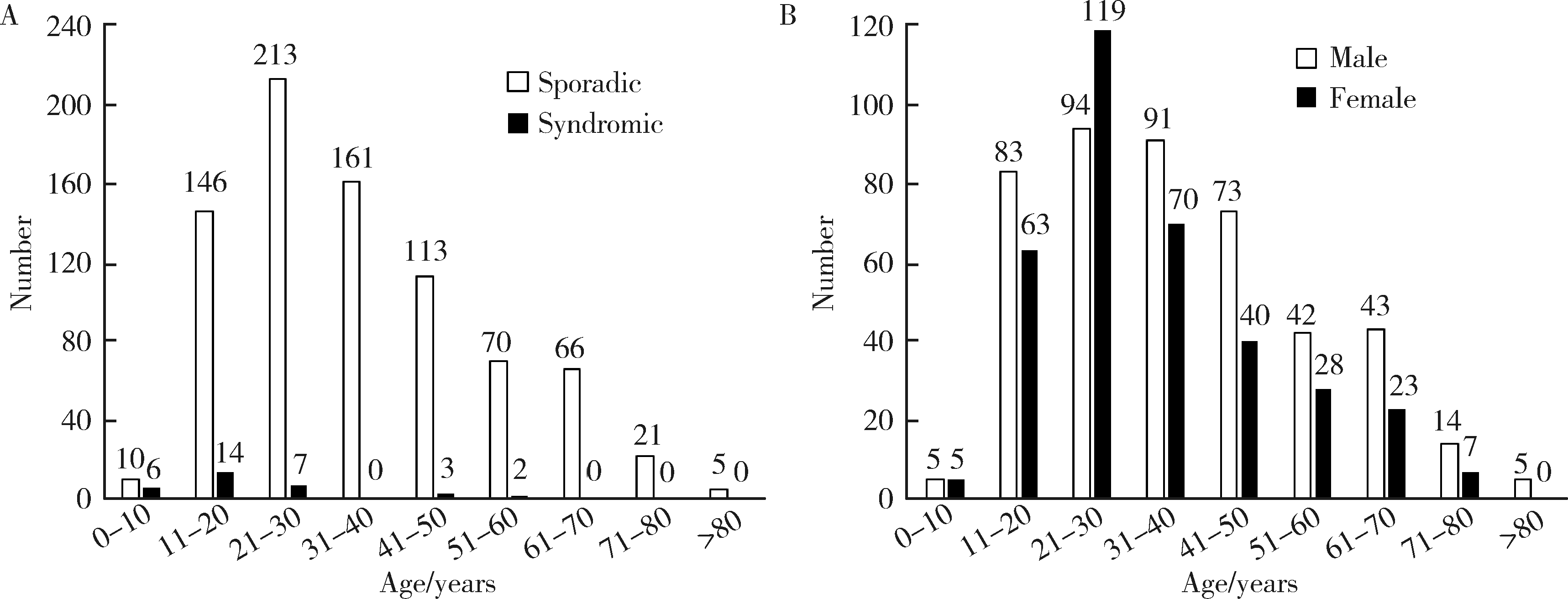
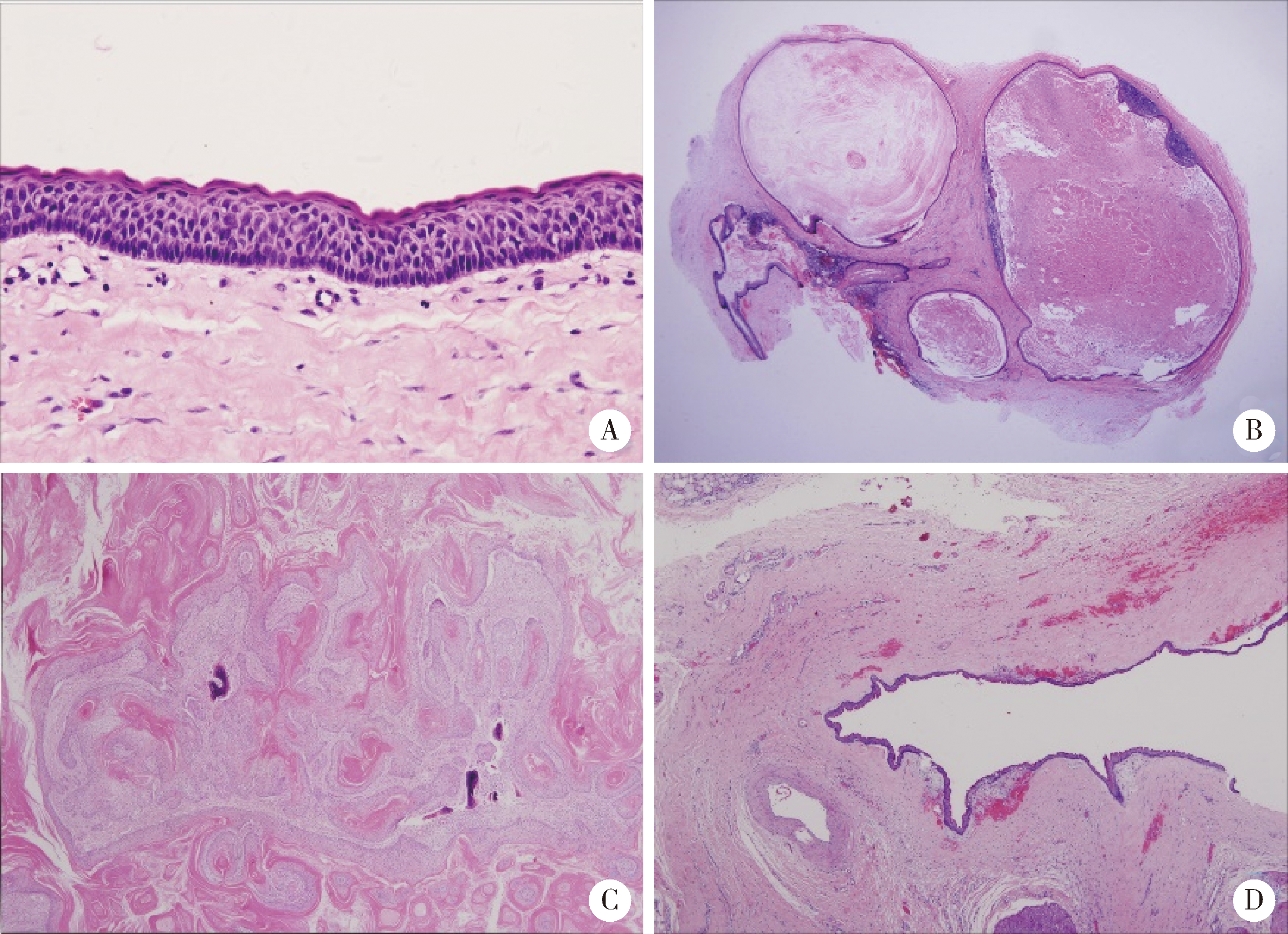
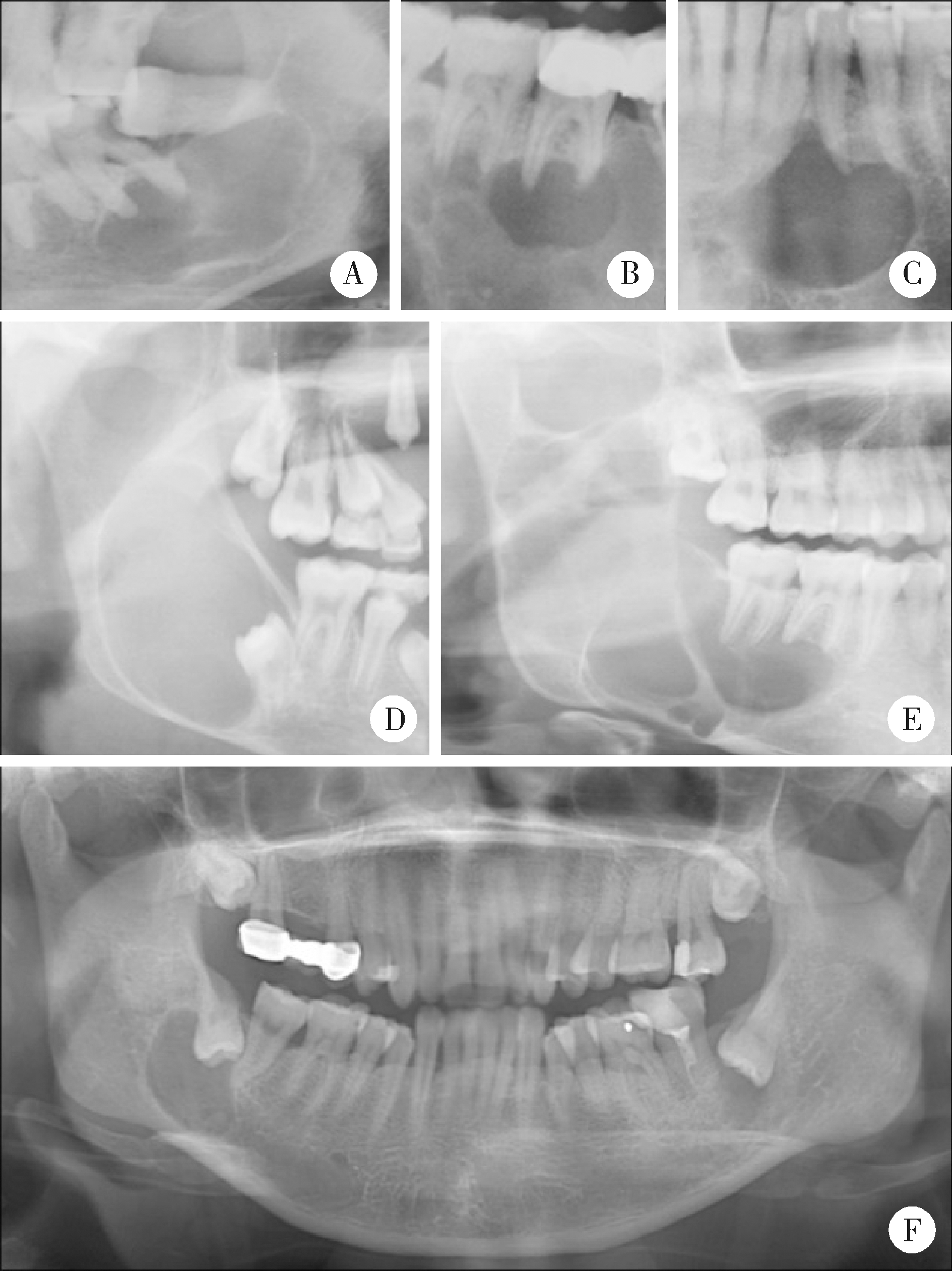
Clinicopathological analysis of 844 cases of odontogenic keratocysts
Yan-jin WANG1,Xiao-yan XIE2,Ying-ying HONG3,Jia-ying BAI1,Jian-yun ZHANG1,△( ),Tie-jun LI1,△(
),Tie-jun LI1,△( )
)
- 1. Peking University School and Hospital of Stomatology & Department of Oral Pathology, Beijing 100081, China
2. Peking University School and Hospital of Stomatology & Department of Oral and Maxillofacial Radiology, Beijing 100081, China
3. First Clinical Division, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R739.8
| [1] | El-Naggar A, Chan J, Grandis JR , et al. WHO classification of head and neck tumors[M]. 4th ed. Lyon, France: IARC, 2017: 235-236. |
| [2] | Stoelinga PJW . Keratocystic odontogenic tumour (KCOT) has again been renamed odontogenic keratocyst (OKC)[J]. Int J Oral Maxillofac Surg, 2019,48(3):415-416. |
| [3] | Li TJ . The odontogenic keratocyst: a cyst, or a cystic neoplasm?[J]. J Dent Res, 2011,90(2):133-142. |
| [4] | Bresler SC, Padwa BL, Granter SR . Nevoid basal cell carcinoma syndrome (Gorlin syndrome)[J]. Head Neck Pathol, 2016,10(2):119-124. |
| [5] | Jawa DS, Sircar K, Somani R , et al. Gorlin-Goltz syndrome[J]. J Oral Maxillofac Pathol, 2009,13(2):89-92. |
| [6] | Kawano K, Okamura K, Kashima K , et al. Solid variant of keratocystic odontogenic tumor of the mandible: report of a case with a clear cell component and review of the literature[J]. Oral Surg Oral Med Oral Pathol Oral Radiol, 2013,116(5):e393-398. |
| [7] | Chi AC, Owings JR Jr, Muller S . Peripheral odontogenic keratocyst: report of two cases and review of the literature[J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 2005,99(1):71-78. |
| [8] | Luo HY, Li TJ . Odontogenic tumors: a study of 1309 cases in a Chinese population[J]. Oral Oncol, 2009,45(8):706-711. |
| [9] | Gorlin RJ . Nevoid basal cell carcinoma syndrome[J]. Dermatol Clin, 1995,13(1):113-125. |
| [10] | Slusarenko da Silva Y, Stoelinga PJW, Naclério-Homem MDG . Recurrence of nonsyndromic odontogenic keratocyst after marsu-pialization and delayed enucleation vs. enucleation alone: a systematic review and meta-analysis[J]. Oral Maxillofac Surg, 2019,23(1):1-11. |
| [11] | Fidele NB, Yueyu Z, Zhao Y , et al. Recurrence of odontogenic keratocysts and possible prognostic factors: Review of 455 patients[J]. Med Oral Patol Oral Cir Bucal, 2019,24(4):e491-e501. |
| [12] | Stoelinga PJ . Long-term follow-up on keratocysts treated according to a defined protocol[J]. Int J Oral Maxillofac Surg, 2001,30(1):14-25. |
| [13] | Cunha JF, Gomes CC, de Mesquita RA , et al. Clinicopathologic features associated with recurrence of the odontogenic keratocyst: a cohort retrospective analysis[J]. Oral Surg Oral Med Oral Pathol Oral Radiol, 2016,121(6):629-635. |
| [14] | 李铁军 . 牙源性角化囊肿的生长与行为[J]. 中华口腔医学杂志, 2000, ( 04):66-68. |
| [15] | Sigua-Rodriguez EA, Goulart DR, Sverzut A , et al. Is surgical treatment based on a 1-step or 2-step protocol effective in managing the odontogenic keratocyst? [J]. J Oral Maxillofac Surg, 2019, 77(6): 1210. e1-1210. e7. |
| [16] | 陶谦, 兰天俊 . 开窗治疗颌骨囊性病变的临床思考与循证[J]. 口腔疾病防治, 2018,26(12):759-765. |
| [17] | Al-Moraissi EA, Dahan AA, Alwadeai MS , et al. What surgical treatment has the lowest recurrence rate following the management of keratocystic odontogenic tumor?: A large systematic review and meta-analysis[J]. J Craniomaxillofac Surg, 2017,45(1):131-144. |
| [18] | Vazquez-Romero MD, Serrera-Figallo ML, Alberdi-Navarro J , et al. Maxillary peripheral keratocystic odontogenic tumor. A clinical case report[J]. J Clin Exp Dent, 2017,9(1):e167-e171. |
| [1] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [2] | Shuai LIU,Lei LIU,Zhuo LIU,Fan ZHANG,Lulin MA,Xiaojun TIAN,Xiaofei HOU,Guoliang WANG,Lei ZHAO,Shudong ZHANG. Clinical treatment and prognosis of adrenocortical carcinoma with venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 624-630. |
| [3] | Le YU,Shaohui DENG,Fan ZHANG,Ye YAN,Jianfei YE,Shudong ZHANG. Clinicopathological characteristics and prognosis of multilocular cystic renal neoplasm of low malignant potential [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 661-666. |
| [4] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [5] | Yangyi FANG,Qiang LI,Zhigao HUANG,Min LU,Kai HONG,Shudong ZHANG. Well-differentiated papillary mesothelial tumour of the tunica vaginalis: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 741-744. |
| [6] | Yuanyuan ZENG,Yun XIE,Daonan CHEN,Ruilan WANG. Related factors of euthyroid sick syndrome in patients with sepsis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 526-532. |
| [7] | Jian-bin LI,Meng-na LYU,Qiang CHI,Yi-lin PENG,Peng-cheng LIU,Rui WU. Early prediction of severe COVID-19 in patients with Sjögren’s syndrome [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1007-1012. |
| [8] | Huan-rui LIU,Xiang PENG,Sen-lin LI,Xin GOU. Risk modeling based on HER-2 related genes for bladder cancer survival prognosis assessment [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 793-801. |
| [9] | Zi-xuan XUE,Shi-ying TANG,Min QIU,Cheng LIU,Xiao-jun TIAN,Min LU,Jing-han DONG,Lu-lin MA,Shu-dong ZHANG. Clinicopathologic features and prognosis of young renal tumors with tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 802-811. |
| [10] | Han LU,Jian-yun ZHANG,Rong YANG,Le XU,Qing-xiang LI,Yu-xing GUO,Chuan-bin GUO. Clinical factors affecting the prognosis of lower gingival squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 702-707. |
| [11] | Yun-fei SHI,Hao-jie WANG,Wei-ping LIU,Lan MI,Meng-ping LONG,Yan-fei LIU,Yu-mei LAI,Li-xin ZHOU,Xin-ting DIAO,Xiang-hong LI. Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 521-529. |
| [12] | Xiao-juan ZHU,Hong ZHANG,Shuang ZHANG,Dong LI,Xin LI,Ling XU,Ting LI. Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 243-253. |
| [13] | Yu-mei LAI,Zhong-wu LI,Huan LI,Yan WU,Yun-fei SHI,Li-xin ZHOU,Yu-tong LOU,Chuan-liang CUI. Clinicopathological features and prognosis of anorectal melanoma: A report of 68 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 262-269. |
| [14] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
| [15] | Qian SU,Xin PENG,Chuan-xiang ZHOU,Guang-yan YU. Clinicopathological characteristics and prognosis of non-Hodgkin lymphoma in oral and maxillofacial regions: An analysis of 369 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 13-21. |
|
||