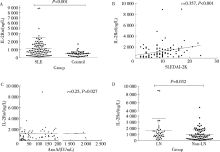
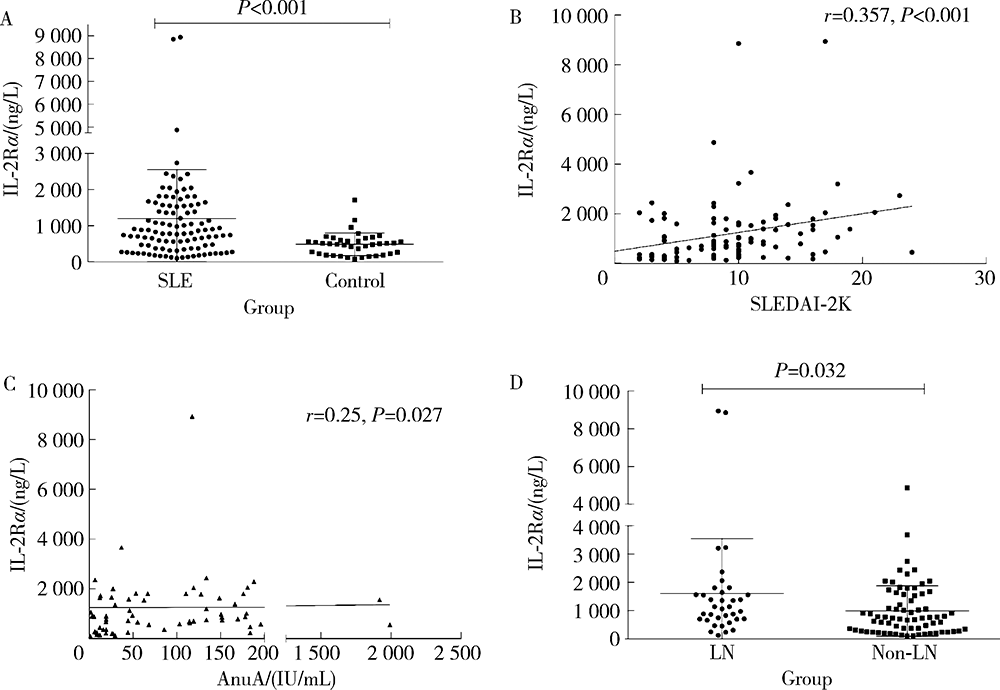
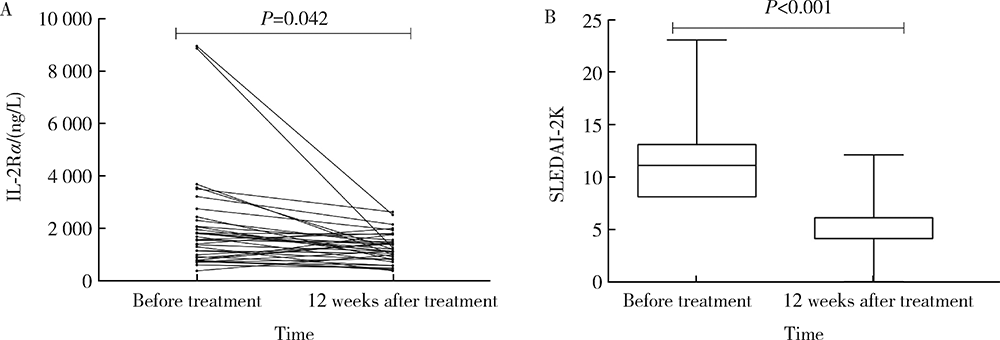
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (6): 1083-1087. doi: 10.19723/j.issn.1671-167X.2021.06.013
Previous Articles Next Articles
Serum interleukin-2 receptor α as a clinical biomarker in patients with systemic lupus erythematosus
TIAN Jia-yi,ZHANG Xia( ),CHENG Gong,LIU Qing-hong,WANG Shi-yang,HE Jing(
),CHENG Gong,LIU Qing-hong,WANG Shi-yang,HE Jing( )
)
- Department of Rheumatology and Immunology, Peking University People’s Hospital, Beijing 100044, China
CLC Number:
- R593.24
| [1] |
Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus[J]. Nat Immunol, 2020, 21(6):605-614.
doi: 10.1038/s41590-020-0677-6 |
| [2] |
Ross SH, Cantrell DA. Signaling and function of interleukin-2 in T lymphocytes[J]. Annu Rev Immunol, 2018, 36:411-433.
doi: 10.1146/immunol.2018.36.issue-1 |
| [3] | He J, Zhang R, Shao M, et al. Efficacy and safety of low-dose IL-2 in the treatment of systemic lupus erythematosus: A randomised, double-blind, placebo-controlled trial[J]. Ann Rheum Dis, 2020, 79(1):141-149. |
| [4] |
He J, Zhang X, Wei Y, et al. Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus[J]. Nat Med, 2016, 22(9):991-993.
doi: 10.1038/nm.4148 |
| [5] |
Rubin L A, Galli F, Greene WC, et al. The molecular basis for the generation of the human soluble interleukin 2 receptor[J]. Cytokine, 1990, 2(5):330-336.
pmid: 2103332 |
| [6] | Dik WA, Heron M. Clinical significance of soluble interleukin-2 receptor measurement in immune-mediated diseases[J]. Neth J Med, 2020, 78(5):220-231. |
| [7] |
Luo H, Wang C, Feng M, et al. Microgravity inhibits resting T cell immunity in an exposure time-dependent manner[J]. Int J Med Sci, 2014, 11(1):87-96.
doi: 10.7150/ijms.7651 |
| [8] |
El-Shafey EM, El-Nagar GF, El-Bendary AS, et al. Serum soluble interleukin-2 receptor alpha in systemic lupus erythematosus[J]. Iran J Kidney Dis, 2008, 2(2):80-85.
pmid: 19377213 |
| [9] | Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus[J]. Arthritis Rheum, 1997, 40(9):1725. |
| [10] |
Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000[J]. J Rheumatol, 2002, 29(2):288-291.
pmid: 11838846 |
| [11] |
Romero-Diaz J, Isenberg D, Ramsey-Goldman R. Measures of adult systemic lupus erythematosus: Updated version of British Isles Lupus Assessment Group (BILAG 2004), European Consensus Lupus Activity Measurements (ECLAM), Systemic Lupus Activity Measure, Revised (SLAM-R), Systemic Lupus Activity Questionnaire for Population Studies (SLAQ), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI)[J]. Arthritis Care Res (Hoboken), 2011, 63(Suppl 11):S37-S46.
doi: 10.1002/acr.v63.11s |
| [12] |
Russell SE, Moore AC, Fallon PG, et al. Soluble IL-2Rα (sCD25) exacerbates autoimmunity and enhances the development of Th17 responses in mice[J]. PLoS One, 2012, 7(10):e47748.
doi: 10.1371/journal.pone.0047748 |
| [13] | Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: From molecular mechanisms to human therapy[J]. Nat Rev Immunol, 2018, 18(10):648-659. |
| [14] | Mizui M, Tsokos GC. Targeting regulatory T cells to treat patients with systemic lupus erythematosus[J]. Front Immunol, 2018(9):786. |
| [15] |
Laut J, Senitzer D, Petrucci R, et al. Soluble interleukin-2 receptor levels in lupus nephritis[J]. Clin Nephrol, 1992, 38(4):179-184.
pmid: 1424303 |
| [1] | Jiang JIN, Xue CHEN, Yan ZHAO, Jun JIA, Jianzhong ZHANG. The role and its regulatory significance of interleukin-25 in ovalbumin induced atopic dermatitis of mice [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 756-762. |
| [2] | Ying TANG, Yongbo ZHANG, Danhong WU, Yanhong LIN, Fenghua LAN. Detection of pathogenic gene mutations in thirteen cases of congenital bilateral absence of vas deferens infertility patients [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 763-774. |
| [3] | Zhihui WU, Mingzhi HU, Qiaoying ZHAO, Fengfeng LV, Jingying ZHANG, Wei ZHANG, Yongfu WANG, Xiaolin SUN, Hui WANG. Immunomodulatory mechanism of umbilical cord mesenchymal stem cells modified by miR-125b-5p in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 860-867. |
| [4] | Xiaodong CHAI,Ziwen SUN,Haishuang LI,Liangyi ZHU,Xiaodan LIU,Yantao LIU,Fei PEI,Qing CHANG. Clinicopathological characteristics of the CD8+ T lymphocytes infiltration and its mechanism in distinct molecular subtype of medulloblastoma [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 512-518. |
| [5] | Limin REN,Chuchu ZHAO,Yi ZHAO,Huiqiong ZHOU,Liyun ZHANG,Youlian WANG,Lingxun SHEN,Wenqiang FAN,Yang LI,Xiaomei LI,Jibo WANG,Yongjing CHENG,Jiajing PENG,Xiaozhen ZHAO,Miao SHAO,Ru Li. Low disease activity and remission status of systemic lupus erythematosus in a real-world study [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 273-278. |
| [6] | Wen-gen LI,Xiao-dong GU,Rui-qiang WENG,Su-dong LIU,Chao CHEN. Expression and clinical significance of plasma exosomal miR-34-5p and miR-142-3p in systemic sclerosis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1022-1027. |
| [7] | Zhi-jun LUO,Jia-jia WU,You SONG,Chun-li MEI,Rong DU. Systemic lupus erythematosus associated macrophage activation syndrome with neuropsychiatric symptoms: A report of 2 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1111-1117. |
| [8] | Hai-hong YAO,Fan YANG,Su-mei TANG,Xia ZHANG,Jing HE,Yuan JIA. Clinical characteristics and diagnostic indicators of macrophage activation syndrome in patients with systemic lupus erythematosus and adult-onset Still's disease [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 966-974. |
| [9] | Xiang-ge ZHAO,Jia-qing LIU,Hui-na HUANG,Zhi-min LU,Zi-ran BAI,Xia LI,Jing-jing QI. Interferon-α mediating the functional damage of CD56dimCD57+natural killer cells in peripheral blood of systemic lupus erythematosuss [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 975-981. |
| [10] | Zhuo-hua LIN,Ru-yi CAI,Yang SUN,Rong MU,Li-gang CUI. Methodology and clinical use of superb microvascular imaging in assessing micro-circulation changes of fingertips in systemic sclerosis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 636-640. |
| [11] | Xiao-juan ZHU,Hong ZHANG,Shuang ZHANG,Dong LI,Xin LI,Ling XU,Ting LI. Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 243-253. |
| [12] | Fang CHENG,Shao-ying YANG,Xing-xing FANG,Xuan WANG,Fu-tao ZHAO. Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1074-1078. |
| [13] | Lu ZHANG,Cheng CHEN,Mei-ting WENG,Ai-ping ZHENG,Mei-ling SU,Qing-wen WANG,Yue-ming CAI. Characteristics of serum autoantibodies in patients with lupus nephritis and tubulointerstitial damage [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1094-1098. |
| [14] | Lin-qi ZHANG,Jing ZHAO,Hong-yan WANG,Zong-yi WANG,Ying-ni LI,Ji-yang TANG,Si-ying LI,Jin-feng QU,Ming-wei ZHAO. Relationship between anti-ENO1 antibody and systemic lupus erythematosus patients with retinopathy [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1099-1105. |
| [15] | Min LI,Lin-qing HOU,Yue-bo JIN,Jing HE. Clinical and immunological characteristics of systemic lupus erythematosus with retinopathy [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1106-1111. |
|
||