Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (2): 320-326. doi: 10.19723/j.issn.1671-167X.2022.02.020
Previous Articles Next Articles
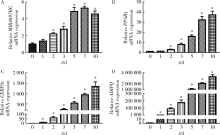
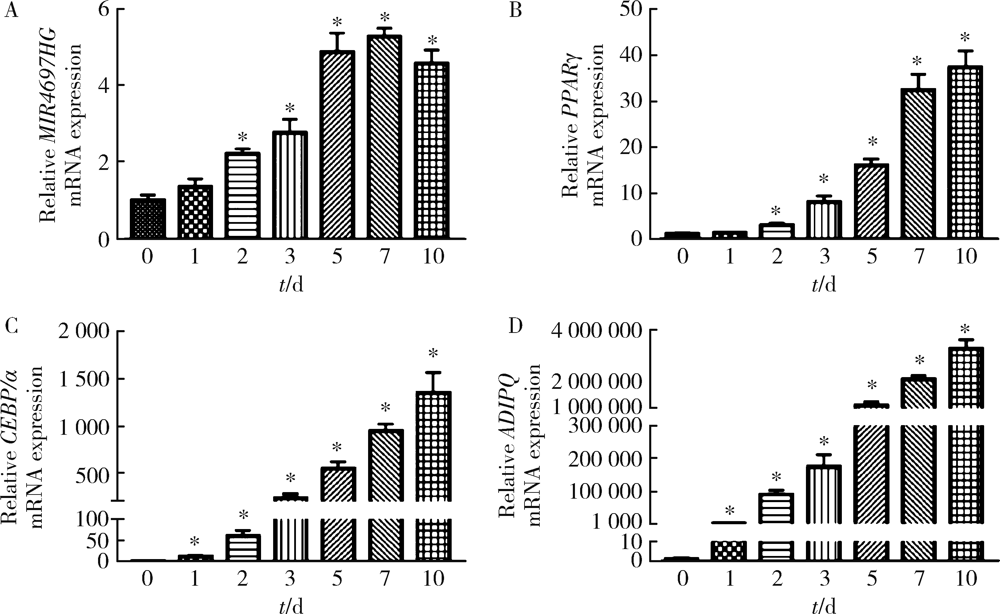
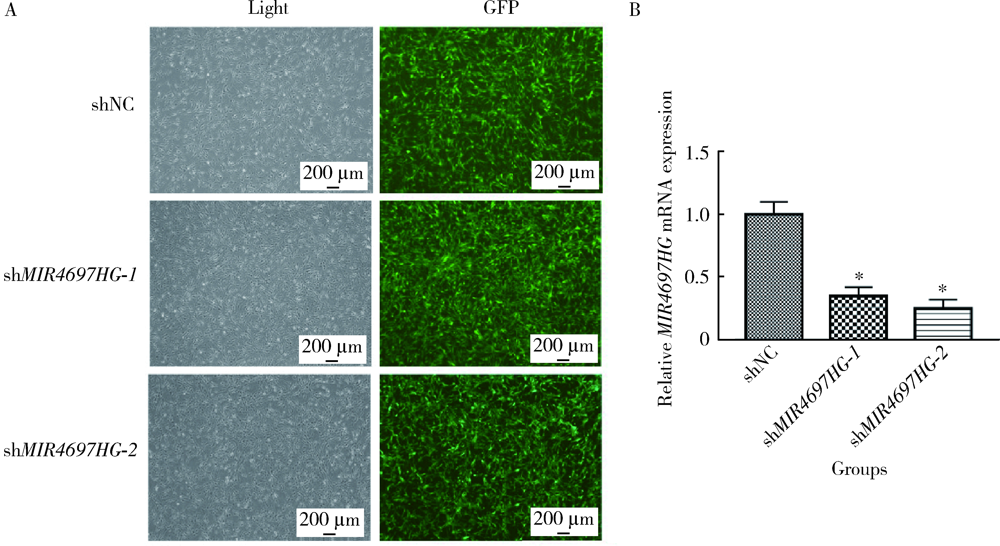
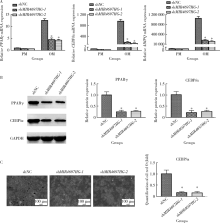
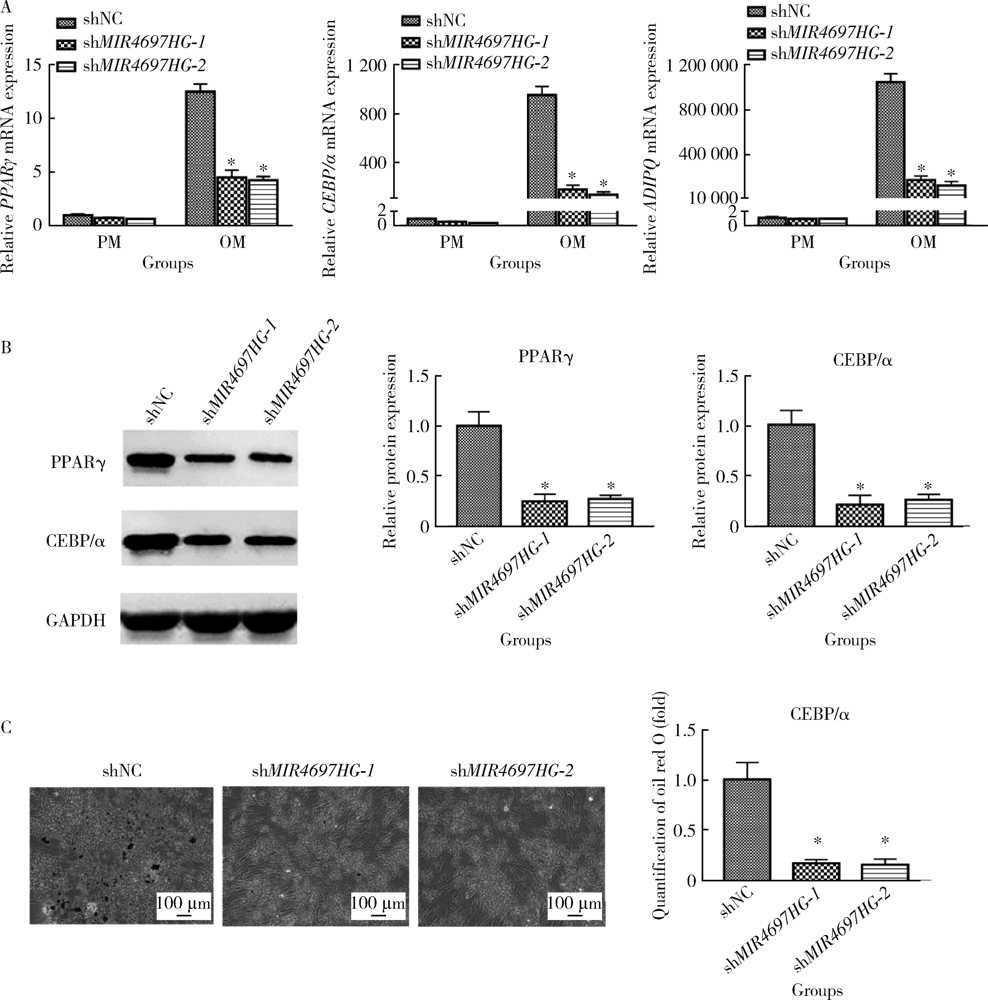
Knockdown of long non-coding RNA MIR4697 host gene inhibits adipogenic differentiation in bone marrow mesenchymal stem cells
SHUAI Ting1,LIU Juan2,GUO Yan-yan1,JIN Chan-yuan1,△( )
)
- 1. Second Clinical Division, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices & Beijing Key Laboratory of Digital Stomatology & NHC Research Center of Engineering and Technology for Computerized Dentistry & NMPA Key Laboratory for Dental Materials, Beijing 100081, China
2. Department of Stomatology, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
CLC Number:
- R34
| [1] |
Pino AM, Rosen CJ, Rodríguez JP. In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis[J]. Biol Res, 2012, 45(3):279-287.
doi: 10.4067/S0716-97602012000300009 |
| [2] |
Taylor DH, Chu TJ, Spektor R, et al. Long non-coding RNA regulation of reproduction and development[J]. Mol Reprod Dev, 2015, 82(12):932-956.
doi: 10.1002/mrd.22581 pmid: 26517592 |
| [3] |
Sun M, Kraus WL. From discovery to function: the expanding roles of long non-coding RNAs in physiology and disease[J]. Endocr Rev, 2015, 36(1):25-64.
doi: 10.1210/er.2014-1034 |
| [4] |
Luan A, Paik KJ, Li J, et al. RNA sequencing for identification of differentially expressed noncoding transcripts during adipogenic differentiation of adipose-derived stromal cells[J]. Plast Reconstr Surg, 2015, 136(4):752-763.
doi: 10.1097/PRS.0000000000001582 |
| [5] |
Liu Y, Wang Y, He X, et al. LncRNA TINCR/miR-31-5p/C/EBP-α feedback loop modulates the adipogenic differentiation process in human adipose tissue-derived mesenchymal stem cells[J]. Stem Cell Res, 2018, 32:35-42.
doi: 10.1016/j.scr.2018.08.016 |
| [6] |
Mao YH, Shen G, Su ZP, et al. RAD21 inhibited transcription of tumor suppressor MIR4697HG and led to glioma tumorigenesis[J]. Biomed Pharmacother, 2020, 123:109759.
doi: 10.1016/j.biopha.2019.109759 |
| [7] | Zhang LQ, Yang SQ, Wang Y, et al. Long noncoding RNA MIR4697HG promotes cell growth and metastasis in human ovarian cancer[J]. Anal Cell Pathol (Amst), 2017, 2017(4):1-9. |
| [8] |
Park SB, Kim J, Jeong JH, et al. Prevalence and incidence of osteoporosis and osteoporotic vertebral fracture in Korea: nationwide epidemiological study focusing on differences in socioecono-mic status[J]. Spine, 2016, 41(4):328.
doi: 10.1097/BRS.0000000000001291 |
| [9] |
Montagner RLM, Martinez CR, Canterle DOL, et al. Factors related with osteoporosis treatment in postmenopausal women[J]. Medicine, 2018, 97(28):e11524.
doi: 10.1097/MD.0000000000011524 |
| [10] |
Zhou X, Liu Z, Huang B, et al. Orcinol glucoside facilitates the shift of MSC fate to osteoblast and prevents adipogenesis via Wnt/beta-catenin signaling pathway[J]. Drug Des Devel Ther, 2019, 13:2703-2713.
doi: 10.2147/DDDT |
| [11] |
Crockett JC, Mellis DJ, Scott DI, et al. New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: focus on the RANK/RANKL axis[J]. Osteoporos Int, 2011, 22(1):1-20.
doi: 10.1007/s00198-010-1272-8 pmid: 20458572 |
| [12] |
Lorenzo J. The many ways of osteoclast activation[J]. J Clin Invest, 2017, 127(7):2530-2532.
doi: 10.1172/JCI94606 pmid: 28530641 |
| [13] | Wang QL, Li HF, Wang DP, et al. Effect of GGCX on the differentiation function of osteoporosis bone marrow mesenchymal stem cells through regulating TGFbeta/smad signaling pathway[J]. Eur Rev Med Pharmacol Sci, 2019, 23(17):7224-7231. |
| [14] |
Aimaiti A, Wahafu T, Keremu A, et al. Strontium ameliorates glucocorticoid inhibition of osteogenesis via the ERK signaling pathway[J]. Biol Trace Elem Res, 2020, 197(2):591-598.
doi: 10.1007/s12011-019-02009-6 |
| [15] |
Chen X, Zhi X, Yin Z, et al. 18β-glycyrrhetinic acid inhibits osteoclastogenesis in vivo and in vitro by blocking RANKL-mediated RANK-TRAF6 interactions and NF-κB and MAPK signaling pathways[J]. Front Pharmacol, 2018, 9:647.
doi: 10.3389/fphar.2018.00647 |
| [16] | Chen X, He F, Zhong DY, et al. Acoustic-frequency vibratory stimulation regulates the balance between osteogenesis and adipogenesis of human bone marrow-derived mesenchymal stem cells[J]. Biomed Res Int, 2015, 2015:540731. |
| [17] | James AW. Review of signaling pathways governing MSC osteoge-nic and adipogenic differentiation[J]. Scientifica, 2013, 2013:684736. |
| [18] |
Shang GW, Wang YD, Xu Y, et al. Long non-coding RNA TCONS_00041960 enhances osteogenesis and inhibits adipogenesis of rat bone marrow mesenchymal stem cell by targeting miR-204-5p and miR-125a-3p[J]. J Cell Physiol, 2018, 233(8):6041-6051.
doi: 10.1002/jcp.v233.8 |
| [19] |
Wang YJ, Liu WT, Liu YD, et al. Long noncoding RNA H19 mediates LCoR to impact the osteogenic and adipogenic differentiation of mBMSCs in mice through sponging miR-188[J]. J Cell Physiol, 2018, 233(9):7435-7446.
doi: 10.1002/jcp.v233.9 |
| [20] |
Yang L, Li Y, Gong R, et al. The long non-coding RNA-ORLNC1 regulates bone mass by directing mesenchymal stem cell fate[J]. Mol Ther, 2019, 27(2):394-410.
doi: S1525-0016(18)30586-0 pmid: 30638773 |
| [21] |
Yuan HR, Xu XW, Feng X, et al. A novel long noncoding RNA PGC1β-OT1 regulates adipocyte and osteoblast differentiation through antagonizing miR-148a-3p[J]. Cell Death Differ, 2019, 26(10):2029-2045.
doi: 10.1038/s41418-019-0296-7 |
| [1] | Xiang-song BAI,Long-wei LV,Yong-sheng ZHOU. Tribbles pseudokinase 3 inhibits the adipogenic differentiation of human adipose-derived mesenchymal stem cells [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 1-9. |
| [2] | Jing XIE,Yu-ming ZHAO,Nan-quan RAO,Xiao-tong WANG,Teng-jiao-zi FANG,Xiao-xia LI,Yue ZHAI,Jing-zhi LI,Li-hong GE,Yuan-yuan WANG. Comparative study of differentiation potential of mesenchymal stem cells derived from orofacial system into vascular endothelial cells [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 900-906. |
| [3] | Fei-long YANG,Kai HONG,Guo-jiang ZHAO,Cheng LIU,Yi-meng SONG,Lu-lin MA. Construction of prognostic model and identification of prognostic biomarkers based on the expression of long non-coding RNA in bladder cancer via bioinformatics [J]. Journal of Peking University(Health Sciences), 2019, 51(4): 615-622. |
| [4] | Xia LIU,Ying ni LI,Xiao li SUN,Qing lin PENG,Xin LU,Guo chun WANG. Effects of integrin metalloproteinases on osteogenic differentiation [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 962-967. |
|
||