Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (3): 505-511. doi: 10.19723/j.issn.1671-167X.2024.03.018
Previous Articles Next Articles
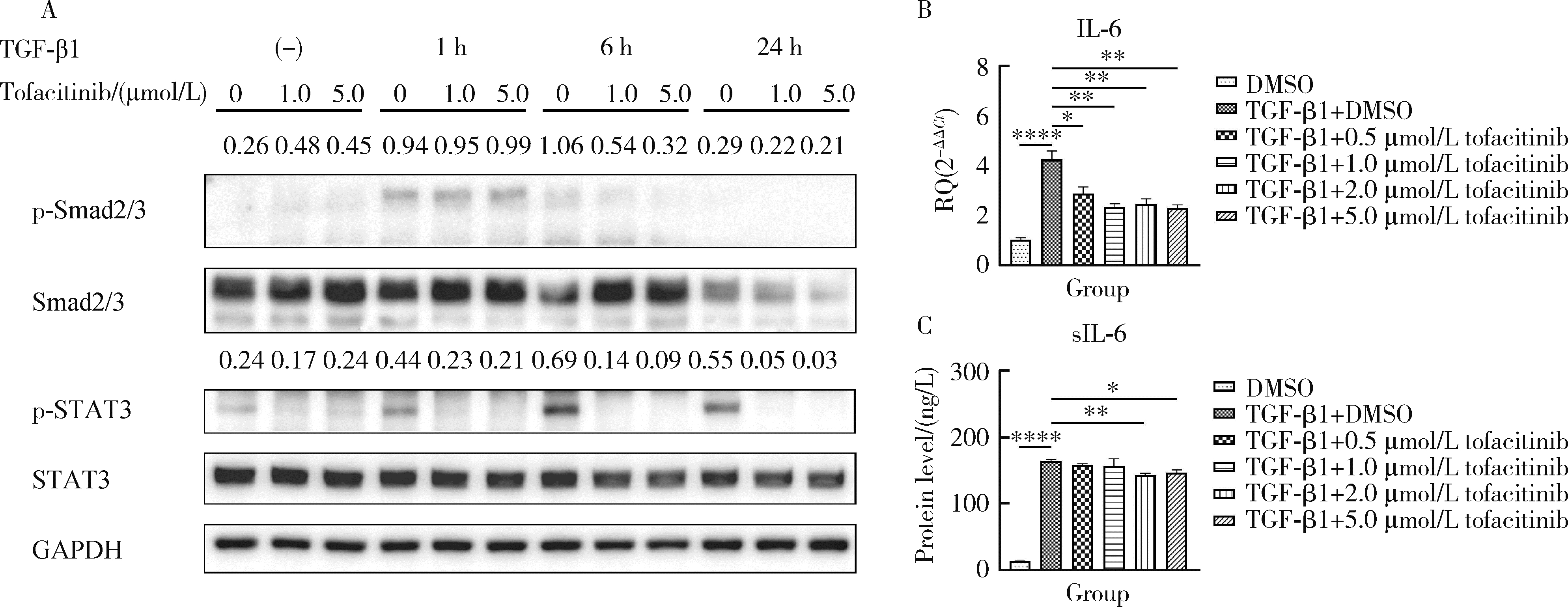
Tofacitinib inhibits the transformation of lung fibroblasts into myofibroblasts through JAK/STAT3 pathway
Shan HE1,2,Xin CHEN1,2,Qi CHENG1,Lingjiang ZHU1,Peiyu ZHANG1,Shuting TONG1,Jing XUE1,Yan DU1,*( )
)
- 1. Department of Rheumatology, the Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, 310009, China
2. Department of Rheumatology, the Affiliated Jinhua Hospital of Zhejiang University School of Medicine, Jinhua, 321000, Zhejiang, China
CLC Number:
- R563.13
| 1 |
Shao T , Shi X , Yang S , et al. Interstitial lung disease in connective tissue disease: A common lesion with heterogeneous mechanisms and treatment considerations[J]. Front Immunol, 2021, 12, 684699.
doi: 10.3389/fimmu.2021.684699 |
| 2 |
Cutolo M , Ruaro B , Montagna P , et al. Effects of selexipag and its active metabolite in contrasting the profibrotic myofibroblast activity in cultured scleroderma skin fibroblasts[J]. Arthritis Res Ther, 2018, 20 (1): 77.
doi: 10.1186/s13075-018-1577-0 |
| 3 | Truchetet ME , Brembilla NC , Chizzolini C . Current concepts on the pathogenesis of systemic sclerosis[J]. Clin Rev Allergy Immunol, 2023, 64 (3): 262- 283. |
| 4 |
Finnson KW , Almadani Y , Philip A . Non-canonical (non-SMAD2/3) TGF-β signaling in fibrosis: Mechanisms and targets[J]. Semin Cell Dev Biol, 2020, 101, 115- 122.
doi: 10.1016/j.semcdb.2019.11.013 |
| 5 |
You H , Xu D , Zhao J , et al. JAK inhibitors: Prospects in connective tissue diseases[J]. Clin Rev Allergy Immunol, 2020, 59 (3): 334- 351.
doi: 10.1007/s12016-020-08786-6 |
| 6 |
Wang W , Bhattacharyya S , Marangoni RG , et al. The JAK/STAT pathway is activated in systemic sclerosis and is effectively targeted by tofacitinib[J]. J Scleroderma Relat Disord, 2020, 5 (1): 40- 50.
doi: 10.1177/2397198319865367 |
| 7 |
Yoo H , Hino T , Hwang J , et al. Connective tissue disease-related interstitial lung disease (CTD-ILD) and interstitial lung abnorma-lity (ILA): Evolving concept of CT findings, pathology and management[J]. Eur J Radiol Open, 2022, 9, 100419.
doi: 10.1016/j.ejro.2022.100419 |
| 8 |
Montero P , Milara J , Roger I , et al. Role of JAK/STAT in interstitial lung diseases, molecular and cellular mechanisms[J]. Int J Mol Sci, 2021, 22 (12): 6211.
doi: 10.3390/ijms22126211 |
| 9 |
Lescoat A , Lelong M , Jeljeli M , et al. Combined anti-fibrotic and anti-inflammatory properties of JAK-inhibitors on macrophages in vitro and in vivo: Perspectives for scleroderma-associated interstitial lung disease[J]. Biochem Pharmacol, 2020, 178, 114103.
doi: 10.1016/j.bcp.2020.114103 |
| 10 |
Kagan P , Sultan M , Tachlytski I , et al. Both MAPK and STAT3 signal transduction pathways are necessary for IL-6-dependent hepatic stellate cells activation[J]. PLoS One, 2017, 12 (5): e0176173.
doi: 10.1371/journal.pone.0176173 |
| 11 | Chen W , Li Y , Hsu CT , et al. Connective tissue growth factor in hepatocytes is elevated by carbon tetrachloride via STAT3 activation[J]. Mol Med Rep, 2020, 21 (3): 1390- 1398. |
| 12 |
Dees C , Tomcik M , Palumbo-Zerr K , et al. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor β in systemic sclerosis[J]. Arthritis Rheum, 2012, 64 (9): 3006- 3015.
doi: 10.1002/art.34500 |
| 13 |
Chakraborty D , Šumová B , Mallano T , et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis[J]. Nat Commun, 2017, 8 (1): 1130.
doi: 10.1038/s41467-017-01236-6 |
| 14 |
Li HG , You PT , Xia Y , et al. Yu Gan Long ameliorates hepatic fibrosis by inhibiting PI3K/AKT, Ras/ERK and JAK1/STAT3 signaling pathways in CCl4-induced liver fibrosis rats[J]. Curr Med Sci, 2020, 40 (3): 539- 547.
doi: 10.1007/s11596-020-2211-3 |
| 15 |
Yan J , Zhang Z , Yang J , et al. JAK3/STAT6 stimulates bone marrow-derived fibroblast activation in renal fibrosis[J]. J Am Soc Nephrol, 2015, 26 (12): 3060- 3071.
doi: 10.1681/ASN.2014070717 |
| 16 |
Pedroza M , Le TT , Lewis K , et al. STAT-3 contributes to pulmonary fibrosis through epithelial injury and fibroblast-myofibroblast differentiation[J]. FASEB J, 2016, 30 (1): 129- 140.
doi: 10.1096/fj.15-273953 |
| 17 |
Levine RL , Pardanani A , Tefferi A , et al. Role of JAK2 in the pathogenesis and therapy of myeloproliferative disorders[J]. Nat Rev Cancer, 2007, 7 (9): 673- 683.
doi: 10.1038/nrc2210 |
| [1] | Xue ZOU,Xiao-juan BAI,Li-qing ZHANG. Effectiveness of tofacitinib combined with iguratimod in the treatment of difficult-to-treat moderate-to-severe rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1013-1021. |
| [2] | GAO Hong-yu,MENG Huan-xin,HOU Jian-xia,HUANG Bao-xin,LI Wei. Expression and distribution of calprotectin in healthy and inflamed periodontal tissues [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 744-749. |
| [3] | Bing-qing SHI,Xiao-jing YUAN,Yu-ming ZHAO. Effects of mineral trioxide aggregate and ethanolic extracts of Shandong propolis on the biological properties of human dental pulp fibroblasts [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1108-1114. |
| [4] | Miao ZHENG,Ling-lu ZHAN,Zhi-qiang LIU,He-ping LI,Jian-guo TAN. Effect of different plasma treated zirconia on the adhensive behaviour of human gingival fibroblasts [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 315-320. |
| [5] | JIA Shuang-shuang, LI Wei-yang, LIU Xin, LI Li-ying. Transforming growth factor-β1 induces differentiation of bone marrowderived mesenchymal stem cells into myofibroblasts via production of reactive oxygen species [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 737-742. |
|
||