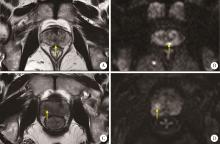
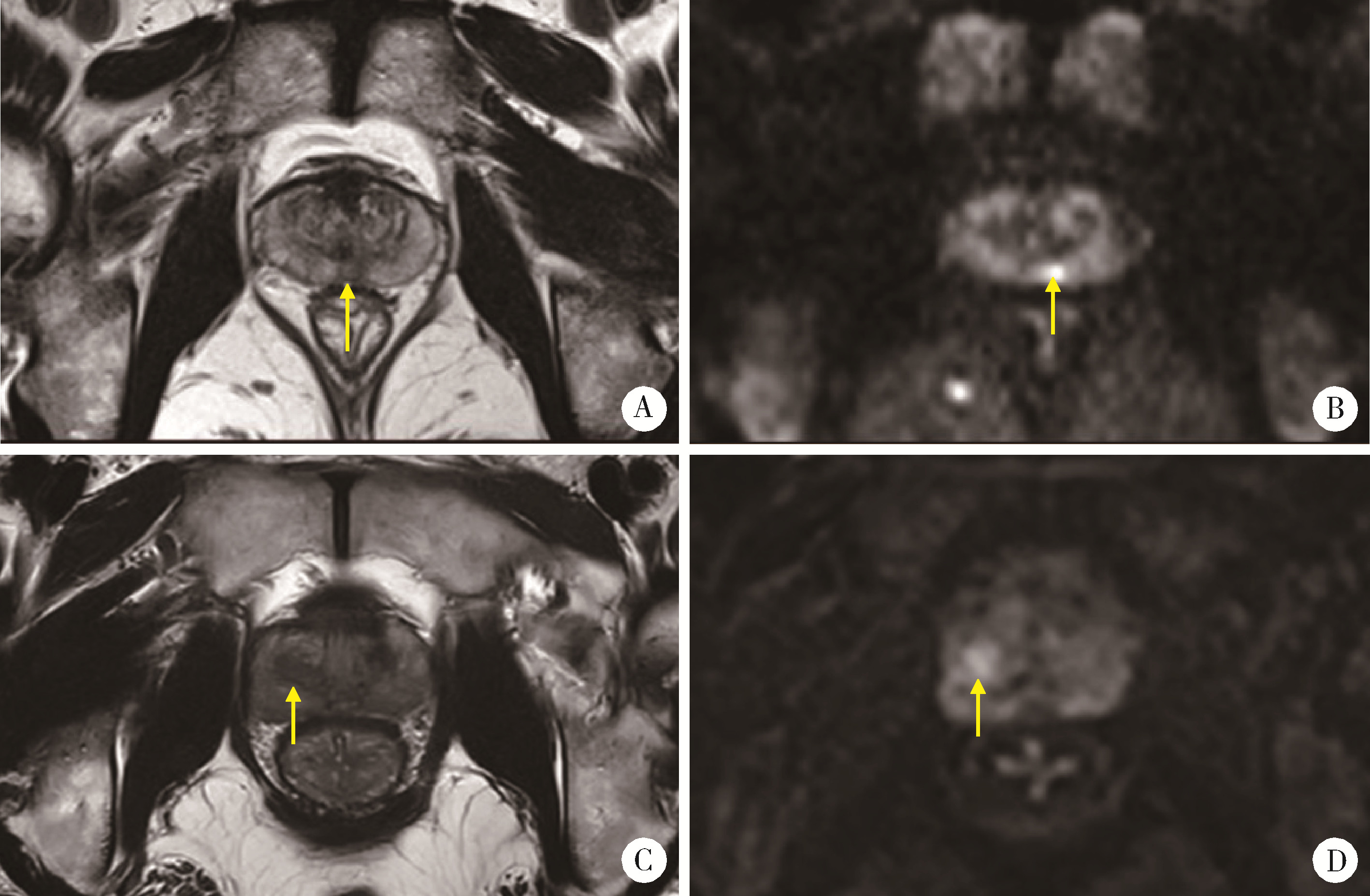
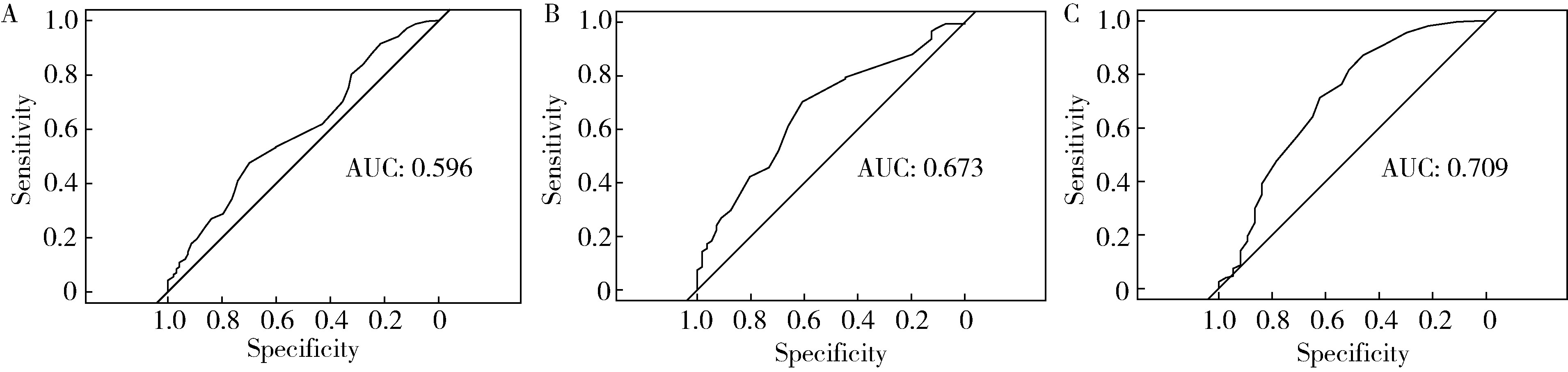
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (4): 567-574. doi: 10.19723/j.issn.1671-167X.2024.04.004
Previous Articles Next Articles
Predictive effect of the dual-parametric MRI modified maximum diameter of the lesions with PI-RADS 4 and 5 on the clinically significant prostate cancer
Yuxuan TIAN1,Mingjian RUAN1,Yi LIU1,Derun LI1,Jingyun WU2,Qi SHEN1,Yu FAN1,3,*( ),Jie JIN1,*(
),Jie JIN1,*( )
)
- 1. Department of Urology, Peking University First Hospital; Institute of Urology, Peking University; National Urological Cancer Center, Beijing 100034, China
2. Department of Radiology, Peking University First Hospital, Beijing 100034, China
3. Drug Clinical Trial Institution, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R737.25
| 1 |
Sung H , Ferlay J , Siegel RL , et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71 (3): 209- 249.
doi: 10.3322/caac.21660 |
| 2 |
Culp MB , Soerjomataram I , Efstathiou JA , et al. Recent global patterns in prostate cancer incidence and mortality rates[J]. Eur Urol, 2020, 77 (1): 38- 52.
doi: 10.1016/j.eururo.2019.08.005 |
| 3 |
Schaeffer EM , Srinivas S , Adra N , et al. Prostate cancer, version 4.2023, NCCN clinical practice guidelines in oncology[J]. J Natl Compr Canc Netw, 2023, 21 (10): 1067- 1096.
doi: 10.6004/jnccn.2023.0050 |
| 4 |
Elkhoury FF , Felker ER , Kwan L , et al. Comparison of targeted vs systematic prostate biopsy in men who are biopsy naive: The prospective assessment of image registration in the diagnosis of prostate cancer (PAIREDCAP) study[J]. JAMA Surg, 2019, 154 (9): 811- 818.
doi: 10.1001/jamasurg.2019.1734 |
| 5 |
Mehralivand S , Bednarova S , Shih JH , et al. Prospective evaluation of PI-RADSTM version 2 using the International Society of Urological Pathology prostate cancer grade group system[J]. J Urol, 2017, 198 (3): 583- 590.
doi: 10.1016/j.juro.2017.03.131 |
| 6 |
Turkbey B , Rosenkrantz AB , Haider MA , et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2[J]. Eur Urol, 2019, 76 (3): 340- 351.
doi: 10.1016/j.eururo.2019.02.033 |
| 7 |
Weinreb JC , Barentsz JO , Choyke PL , et al. PI-RADS Prostate Imaging: Reporting and data system: 2015, version 2[J]. Eur Urol, 2016, 69 (1): 16- 40.
doi: 10.1016/j.eururo.2015.08.052 |
| 8 |
Borkowetz A , Platzek I , Toma M , et al. Direct comparison of multiparametric magnetic resonance imaging (MRI) results with final histopathology in patients with proven prostate cancer in MRI/ultrasonography-fusion biopsy[J]. BJU Int, 2016, 118 (2): 213- 220.
doi: 10.1111/bju.13461 |
| 9 |
Cash H , Maxeiner A , Stephan C , et al. The detection of significant prostate cancer is correlated with the prostate imaging reporting and data system (PI-RADS) in MRI/transrectal ultrasound fusion biopsy[J]. World J Urol, 2016, 34 (4): 525- 532.
doi: 10.1007/s00345-015-1671-8 |
| 10 |
Kasivisvanathan V , Rannikko AS , Borghi M , et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis[J]. N Engl J Med, 2018, 378 (19): 1767- 1777.
doi: 10.1056/NEJMoa1801993 |
| 11 |
Radtke JP , Schwab C , Wolf MB , et al. Multiparametric magnetic resonance imaging (MRI) and MRI-transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen[J]. Eur Urol, 2016, 70 (5): 846- 853.
doi: 10.1016/j.eururo.2015.12.052 |
| 12 |
Turkbey B , Mani H , Aras O , et al. Correlation of magnetic resonance imaging tumor volume with histopathology[J]. J Urol, 2012, 188 (4): 1157- 1163.
doi: 10.1016/j.juro.2012.06.011 |
| 13 |
Nelson BA , Shappell SB , Chang SS , et al. Tumour volume is an independent predictor of prostate-specific antigen recurrence in patients undergoing radical prostatectomy for clinically localized prostate cancer[J]. BJU Int, 2006, 97 (6): 1169- 1172.
doi: 10.1111/j.1464-410X.2006.06148.x |
| 14 |
Ploussard G , Beauval JB , Renard-Penna R , et al. Assessment of the minimal targeted biopsy core number per MRI lesion for improving prostate cancer grading prediction[J]. J Clin Med, 2020, 9 (1): 225.
doi: 10.3390/jcm9010225 |
| 15 |
Rais-Bahrami S , Türkbey B , Rastinehad AR , et al. Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: Recommendations for interval imaging follow-up[J]. Diagn Interv Radiol, 2014, 20 (4): 293- 298.
doi: 10.5152/dir.2014.13319 |
| 16 |
Wolters T , Roobol MJ , van Leeuwen PJ , et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial[J]. J Urol, 2011, 185 (1): 121- 125.
doi: 10.1016/j.juro.2010.08.082 |
| 17 |
Johnson DC , Raman SS , Mirak SA , et al. Detection of individual prostate cancer foci via multiparametric magnetic resonance imaging[J]. Eur Urol, 2019, 75 (5): 712- 720.
doi: 10.1016/j.eururo.2018.11.031 |
| 18 |
Matoso A , Epstein JI . Defining clinically significant prostate cancer on the basis of pathological findings[J]. Histopathology, 2019, 74 (1): 135- 145.
doi: 10.1111/his.13712 |
| 19 |
Mahjoub S , Baur ADJ , Lenk J , et al. Optimizing size thresholds for detection of clinically significant prostate cancer on MRI: Peripheral zone cancers are smaller and more predictable than transition zone tumors[J]. Eur J Radiol, 2020, 129, 109071.
doi: 10.1016/j.ejrad.2020.109071 |
| 20 |
Sakala MD , Dyer RB , Tappouni R . The "erased charcoal" sign[J]. Abdom Radiol (NY), 2017, 42 (3): 981- 982.
doi: 10.1007/s00261-016-0938-x |
| 21 |
Alanee S , Deebajah M , Dabaja A , et al. Utilizing lesion diameter and prostate specific antigen density to decide on magnetic resonance imaging guided confirmatory biopsy of prostate imaging reporting and data system score three lesions in African American prostate cancer patients managed with active surveillance[J]. Int Urol Nephrol, 2022, 54 (4): 799- 803.
doi: 10.1007/s11255-022-03136-1 |
| 22 |
Kilic M , Madendere S , Vural M , et al. The role of the size and number of index lesion in the diagnosis of clinically significant prostate cancer in patients with PI-RADS 4 lesions who underwent in-bore MRI-guided prostate biopsy[J]. World J Urol, 2023, 41 (2): 449- 454.
doi: 10.1007/s00345-022-04274-y |
| 23 |
Park MY , Park KJ , Lim B , et al. Comparison of biopsy strategies for prostate biopsy according to lesion size and PSA density in MRI-directed biopsy pathway[J]. Abdom Radiol (NY), 2020, 45 (12): 4166- 4177.
doi: 10.1007/s00261-020-02667-4 |
| 24 | Senel S , Koudonas A , Uzun E , et al. The value of prostate-specific antigen density in combination with lesion diameter for the accuracy of prostate cancer prediction in prostate imaging-reporting and data system 3 prostate lesions[J]. Urol Int, 2023, 107 (10/11/12): 965- 970. |
| 25 |
Costa DN , Goldberg K , Leon AD , et al. Magnetic resonance imaging-guided in-bore and magnetic resonance imaging-transrectal ultrasound fusion targeted prostate biopsies: An adjusted comparison of clinically significant prostate cancer detection rate[J]. Eur Urol Oncol, 2019, 2 (4): 397- 404.
doi: 10.1016/j.euo.2018.08.022 |
| 26 |
Schoots IG , Padhani AR , Rouvière O , et al. Analysis of magnetic resonance imaging-directed biopsy strategies for changing the paradigm of prostate cancer diagnosis[J]. Eur Urol Oncol, 2020, 3 (1): 32- 41.
doi: 10.1016/j.euo.2019.10.001 |
| [1] | Zhicun LI, Tianyu WU, Lei LIANG, Yu FAN, Yisen MENG, Qian ZHANG. Risk factors analysis and nomogram model construction of postoperative pathological upgrade of prostate cancer patients with single core positive biopsy [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 896-901. |
| [2] | Kaifeng YAO,Mingjian RUAN,Derun LI,Yuxuan TIAN,Yuke CHEN,Yu FAN,Yi LIU. Diagnostic efficacy of targeted biopsy combined with regional systematic biopsy in prostate cancer in patients with PI-RADS 4-5 [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 575-581. |
| [3] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [4] | Yi LIU,Chang-wei YUAN,Jing-yun WU,Qi SHEN,Jiang-xi XIAO,Zheng ZHAO,Xiao-ying WANG,Xue-song LI,Zhi-song HE,Li-qun ZHOU. Diagnostic efficacy of prostate cancer using targeted biopsy with 6-core systematic biopsy for patients with PI-RADS 5 [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 812-817. |
| [5] | Chang-wei YUAN,De-run LI,Zhi-hua LI,Yi LIU,Gang-zhi SHAN,Xue-song LI,Li-qun ZHOU. Application of dynamic contrast enhanced status in multiparametric magnetic resonance imaging for prostatic cancer with PI-RADS 4 lesion [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 838-842. |
| [6] | Yan XIONG,Xin LI,Li LIANG,Dong LI,Li-min YAN,Xue-ying LI,Ji-ting DI,Ting LI. Evaluation of accuracy of pathological diagnosis based on thyroid core needle biopsy [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 234-242. |
| [7] | Dan-feng ZHENG,Jun-yu LI,Jia-xi LI,Ying-shuang ZHANG,Yan-feng ZHONG,Miao YU. Pathologic features of paraspinal muscle biopsies in patients with adolescent idiopathic scoliosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 283-291. |
| [8] | ZHOU Guang-ping,ZHOU Qian-yun,ZHU Ji-hong. A case report of TAFRO syndrome [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 814-817. |
| [9] | ZHANG Lei,LI Guo-liang,DANG Zong-hui, ,A yong,WU Ling-jie,LIU Li-jun. Analysis of bleeding risk in percutaneous renal biopsy in Tibet [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 298-301. |
| [10] | WANG Ying-chun,HUANG Yong-hui,CHANG Hong,YAO Wei,YAN Xiu-e,LI Ke,ZHANG Yao-peng,ZHENG Wei. Characteristics of benign and malignant lesions of ampullary polyps and the accuracy of forceps biopsy [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 204-209. |
| [11] | Yi LIU,Zhi-jian LIU,Qi SHEN,Jing-yun WU,Yu FAN,De-run LI,Wei YU,Zhi-song HE. A clinical analysis of 14 cases of prostatic stromal tumor of uncertain malignant potential [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 621-624. |
| [12] | Yi-chang HAO,Ye YAN,Fan ZHANG,Min QIU,Lang ZHOU,Ke LIU,Jian LU,Chun-lei XIAO,Yi HUANG,Cheng LIU,Lu-lin MA. Surgical strategy selection and experience summary of prostate cancer with positive single needle biopsy [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 625-631. |
| [13] | Ye YAN,Hai-zhui XIA,Xu-sheng LI,Wei HE,Xue-hua ZHU,Zhi-ying ZHANG,Chun-lei XIAO,Yu-qing LIU,Hua HUANG,Liang-hua HE,Jian LU. Application of U-shaped convolutional neural network in auto segmentation and reconstruction of 3D prostate model in laparoscopic prostatectomy navigation [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 596-601. |
| [14] | MAO Jia-ming, LIU De-feng,ZHAO Lian-ming,HONG Kai, ZHANG Li, MA Lu-lin, JIANG Hui, QIAO Jie. Effect of testicular puncture biopsy on the success rate of microdissection testicular sperm extraction for idiopathic non-obstructive azoospermia [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 613-616. |
| [15] | ZHANG Fan, XIAO Chun-lei, ZHANG Shu-dong, HUANG Yi, MA Lu-lin. Relationship between recovery of urinary continence after laparoscopic radical prostatectomy and prostatic volume and intravesical prostatic protursion length [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 621-625. |
|
||