Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (5): 887-892. doi: 10.19723/j.issn.1671-167X.2019.05.016
Previous Articles Next Articles
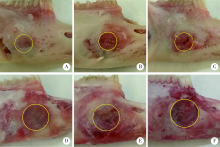
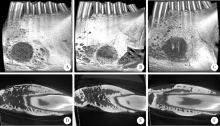
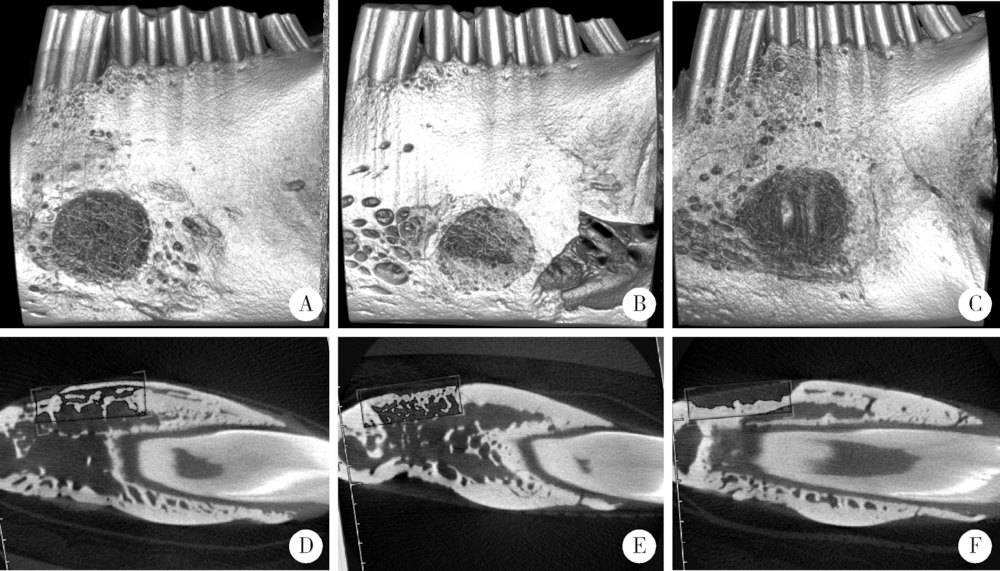
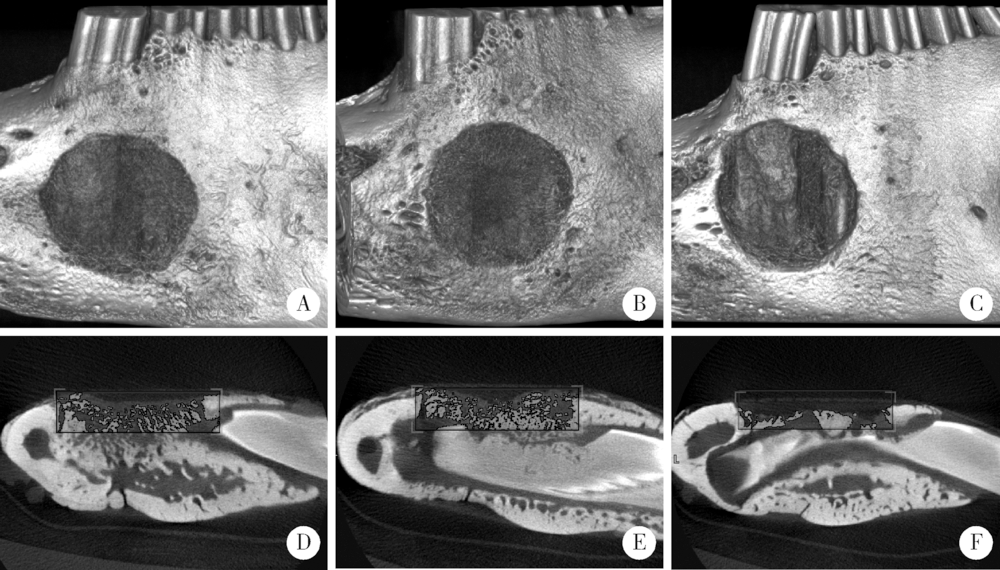
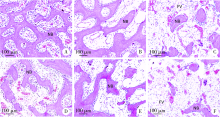
Barrier effect of improved porcine small intestinal submucosa absorbable membrane on early healing of mandibular defects in rabbits
Bo-wen LI,Wei-yi WU,Lin TANG,Yi ZHANG,Yu-hua LIU( )
)
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R738.4
| [1] | Retzepi M, Donos N . Guided bone regeneration: biological principle and therapeutic applications[J]. Clin Oral Implants Res, 2010,21(6):567-576. |
| [2] | Dimitriou R, Mataliotakis GI, Calori GM , et al. The role of bar-rier membranes for guided bone regeneration and restoration of large bone defects: Current experimental and clinical evidence[J]. BMC Med, 2012,10(1):81. |
| [3] | Wessing B, Urban I, Montero E , et al. Guided bone regeneration using collagen membranes simultaneous to implant placement at compromised sites leads to reproducible results and high success rates[J]. Musculoskelet Regen, 2017,3:e1537. |
| [4] | Cen L, Liu W, Cui L , et al. Collagen tissue engineering: Deve-lopment of novel biomaterials and applications[J]. Pediatr Res, 2008,63(5):492-496. |
| [5] | Zitzmann NU, Schärer P, Marinello CP . Long-term results of implants treated with guided bone regeneration: A 5-year prospective study[J]. Int J Oral Maxillofac Implants, 2001,16(3):355-366. |
| [6] | Tal H, Kozlovsky A, Artzi Z , et al. Long-term bio-degradation of cross-linked and non-cross-linked collagen barriers in human guided bone regeneration[J]. Clin Oral Implants Res, 2008,19(3):295-302. |
| [7] | Lee JH, Lee JS, Baek WS , et al. Assessment of dehydrothermally cross-linked collagen membrane for guided bone regeneration around peri-implant dehiscence defects: A randomized single-blinded clinical trial[J]. J Periodontal Implant Sci, 2015,45(6):229-237. |
| [8] | Tu Y, Chen C, Li Y , et al. Fabrication of nano-hydroxyapatite/chitosan membrane with asymmetric structure and its applications in guided bone regeneration[J]. Biomed Mater Eng, 2017,28(3):223-233. |
| [9] | Jiménez GJ, Berghezan S, Jmm C , et al. Effect of cross-linked vs. non-cross-linked collagen membranes on bone: A systematic review[J]. J Periodontal Res, 2017,52(6):955-964. |
| [10] | Andrée B, Bär A, Haverich A , et al. Small intestinal submucosa segments as matrix for tissue engineering: review[J]. Tissue Eng Part B Rev, 2013,19(4):279-291. |
| [11] | Mosala Nezhad Z, Poncelet A, de Kerchove L, et al. Small intestinal submucosa extracellular matrix (CorMatrix ®) in cardiovascular surgery: A systematic review [J]. Interact Cardiovasc Thorac Surg, 2016,22(6):839-850. |
| [12] | Mewaldt R, Shi L, Carson D . Enzymatic degradation study of single layer and multi-layer small intestine submucosa (sis) matrices[J]. Wound Repair Regen, 2011,19(2):A39. |
| [13] | 牛睿, 李智峰, 张海燕 , 等. 复合SIS组织修复材料及其制备方法: 北京, CN103877619A[P]. 2014 -06-25. |
| [14] | 陈薇, 李次会, 武术 , 等. 脱细胞处理对小肠黏膜下层细胞残留及生长因子含量影响的实验研究[J]. 中国修复重建外科志, 2010,24(1):94-99. |
| [15] | Olaechea A, Mendoza-Azpur G, Valdivia E , et al. Biodegradation of three different collagen membranes: A histological study[J]. J Osseointegration, 2016,8(2):15-19. |
| [16] | Gottlow J . Guided tissue regeneration using bioresorbable and non-resorbable devices: Initial healing and long-term results[J]. J Periodontol, 1993,64(11 Suppl):1157-1165. |
| [17] | Behring J, Junker R, Walboomers XF , et al. Toward guided tissue and bone regeneration: morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes: A systematic review[J]. Odontology, 2008,96(1):1-11. |
| [18] | Rothamel D, Schwarz F, Fienitz T , et al. Biocompatibility and biodegradation of a native porcine pericardium membrane: Results of in vitro and in vivo examinations[J]. Int J Oral Maxillofac Implants, 2012,27(1):146-154. |
| [19] | Jung RE, Hälg GA, Thoma DS , et al. A randomized, controlled clinical trial to evaluate a new membrane for guided bone regeneration around dental implants[J]. Clin Oral Implants Res, 2009,20(2):162-168. |
| [20] | de Santana RB, de Mattos CML, Francischone CE , et al. Superficial topography and porosity of an absorbable barrier membrane impacts soft tissue response in guided bone regeneration[J]. J Periodontol, 2010,81(6):926-933. |
| [21] | McPherson TB, Badylak SF . Characterization of fibronectin derived from porcine small intestinal submucosa[J]. Tissue Engineering, 1998,4(1):75-83. |
| [22] | Lindberg K, Badylak SF . Porcine small intestinal submucosa (SIS): A bioscaffold supporting in vitro primary human epidermal cell differentiation and synjournal of basement membrane proteins[J]. Burns, 2001,27(3):254-266. |
| [23] | Ermis R . Comparison of mechanical properties between small intestine submucosa (sis) with varying layers[J]. Wound Repair Regen, 2011,19(2):A21. |
| [24] | 吴唯伊, 李博文, 刘玉华 , 等. 复层猪小肠黏膜下层可吸收膜体内外降解性能的研究[ C]//中华口腔医学会口腔修复学专业委员会. 第十一次全国口腔修复学学术年会论文汇编.北京: 中华口腔医学会口腔修复学专业委员会, 2017: 1. |
| [25] | Schmitz JP, Hollinger JO . The critical size defect as an experimental model for craniomandibulofacial nonunions[J]. Clin Orthop Relat Res, 1986(205):299-308. |
| [26] | Li M, Zhang C, Cheng M , et al. Small intestinal submucosa: A potential osteoconductive and osteoinductive biomaterial for bone tissue engineering[J]. Mater Sci Eng C Mater Appl, 2017,75:149-156. |
| [1] | Xiaoying CHEN,Yi ZHANG,Yuke LI,Lin TANG,Yuhua LIU. Effects of different polymers on biomimetic mineralization of small intestine submucosal scaffolds [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 17-24. |
| [2] | Deng-hui DUAN,Hom-Lay WANG,En-bo WANG. Role of collagen membrane in modified guided bone regeneration surgery using buccal punch flap approach: A retrospective and radiographical cohort study [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1097-1104. |
| [3] | Yu-ke LI,Mei WANG,Lin TANG,Yu-hua LIU,Xiao-ying CHEN. Effect of pH on the chelation between strontium ions and decellularized small intestinal submucosal sponge scaffolds [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 44-51. |
| [4] | Yi DENG,Yi ZHANG,Bo-wen LI,Mei WANG,Lin TANG,Yu-hua LIU. Effects of different crosslinking treatments on the properties of decellularized small intestinal submucosa porous scaffolds [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 557-564. |
| [5] | Mei WANG, Bo-wen LI, Si-wen WANG, Yu-hua LIU. Preparation and osteogenic effect study of small intestinal submucosa sponge [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 952-958. |
| [6] | Wei-yi WU,Bo-wen LI,Yu-hua LIU,Xin-zhi WANG. Biodegradation properties of multi-laminated small intestinal submucosa [J]. Journal of Peking University (Health Sciences), 2020, 52(3): 564-569. |
| [7] | Fei LI,Jing QIAO,Jin-yu DUAN,Yong ZHANG,Xiu-jing WANG. Effect of concentrated growth factors combined with guided tissue regeneration in treatment of classⅡ furcation involvements of mandibular molars [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 346-352. |
| [8] | ZHAN Ya-lin, HU Wen-jie, XU Tao, ZHEN Min, LU Rui-fang. Histomorphometric evaluation of ridge preservation after molar tooth extraction [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 169-175. |
| [9] | QIAO Jing, DUAN Jin-yu, CHU Yi, SUN Chang-zhou. Effect of concentrated growth factors on the treatment of degree Ⅱ furcation involvements of mandibular molars [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 36-042. |
|
||