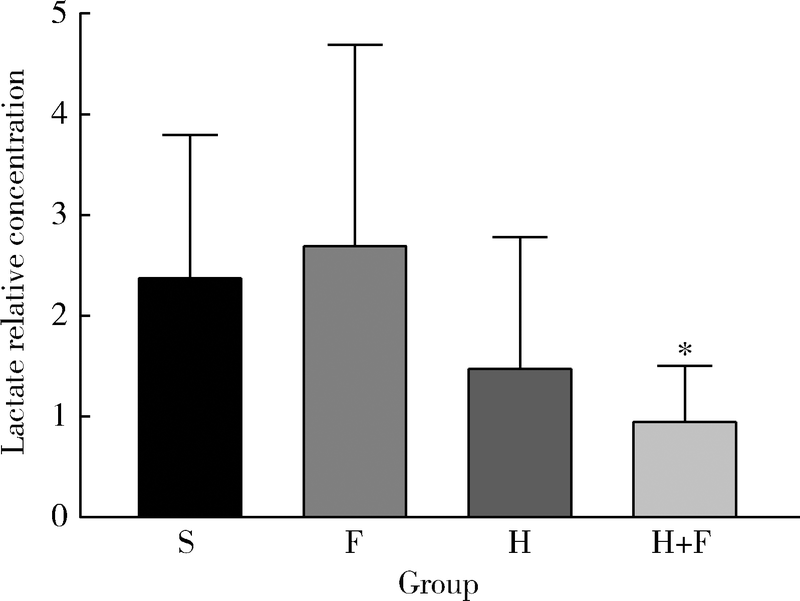
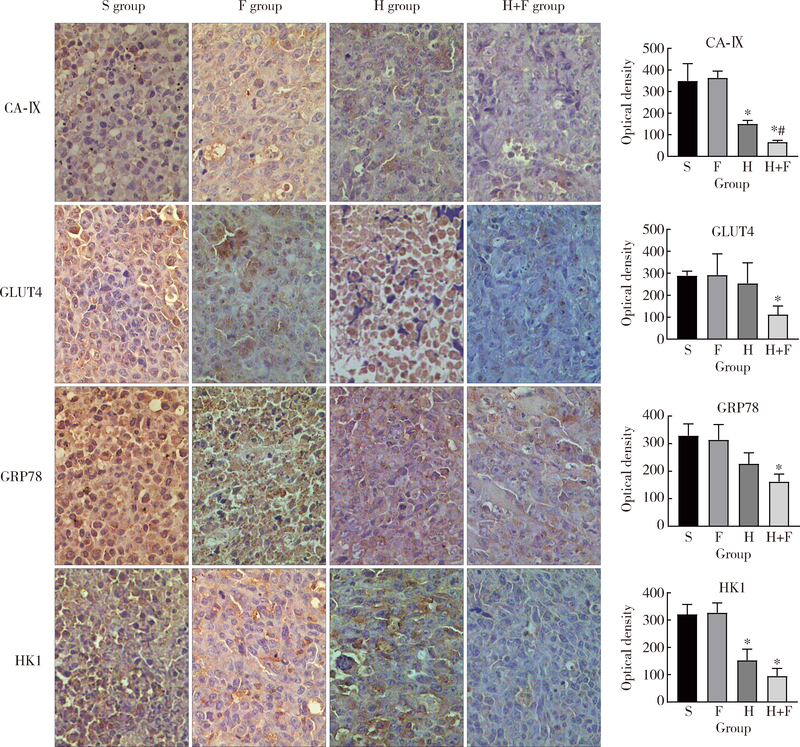
Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (2): 247-253. doi: 10.19723/j.issn.1671-167X.2020.02.009
Previous Articles Next Articles
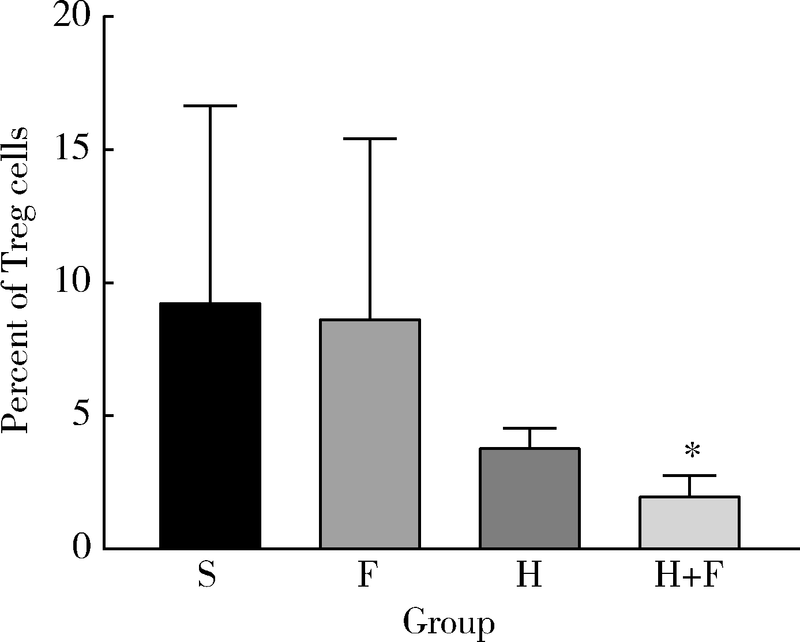
Effect of Fei-Liu-Ping ointment combined with cyclophosphamide on lung cancer cell proliferation and acidic microenvironment
Liang GENG1,Jing LV2,△( ),Jing FAN3
),Jing FAN3
- 1. Department of Chinese and Western Medicine, Cancer Hospital Affiliated to Zhengzhou University, Zhengzhou 450008, China
2. Traditional Chinese Medicine Teaching and Research Office, Zhengzhou Health Vocational College, Zhengzhou 450005, China
3. College of Management, Henan University of Chinese Medicine, Zhengzhou 450008, China
CLC Number:
- R734.2
| [1] | 陈璐, 高威 . 肿瘤酸性微环境的形成机制及其对肿瘤进展的影响[J]. 肿瘤, 2019,39(2):140-145. |
| [2] | Li X, Yu X, Dai D , et al. The altered glucose metabolism in tumor and a tumor acidic microenvironment associated with extracellular matrix metalloproteinase inducer and monocarboxylate transporters[J]. Oncotarget, 2016,7(17):23141-23155. |
| [3] | Wojtkowiak JW, Verduzco D, Schramm KJ , et al. Drug resistance and cellular adaptation to tumor acidic pH microenvironment[J]. Mol Pharm, 2011,8(6):2032-2038. |
| [4] | 龚巍 . 调节性T细胞和胃癌的临床与实验研究[D]. 苏州: 苏州大学, 2017. |
| [5] | Fukumura D, Jain RK . Tumor microvasculature and microenvironment: Targets for anti-angiogenesis and normalization[J]. Microvasc Res, 2007,74(2-3):72-84. |
| [6] | Ganapathy V, Thangaraju M, Prasad PD . Nutrient transporters in cancer: Relevance to Warburg hypojournal and beyond[J]. Pharmacol Ther, 2009,121(1):29-40. |
| [7] | Seagroves TN, Ryan HE, Lu H , et al. Transcription factor HIF-1 is a necessary mediator of the Pasteur effect in mammalian cells[J]. Mol Cell Biol, 2001,21(10):3436-3444. |
| [8] | Semenza GL . Targeting HIF-1 for cancer therapy[J]. Nat Rev Cancer, 2003,3(10):721-732. |
| [9] | Lee JW, Bae SH, Jeong JW , et al. Hypoxia-inducible factor (HIF-1) alpha: Its protein stability and biological functions[J]. Exp Mol Med, 2004,36(1):1-12. |
| [10] | Winum JY, Rami M, Scozzafava A , et al. Carbonic anhydrase Ⅸ: A new druggable target for the design of antitumor agents[J]. Med Res Rev, 2008,28(3):445-463. |
| [11] | van den Beucken T, Ramaekers CH, Rouschop K , et al. Deficient carbonic anhydrase 9 expression in UPR-impaired cells is associated with reduced survival in an acidic microenvironment[J]. Radiother Oncol, 2009,92(3):437-442. |
| [12] | Verras M, Papandreou I, Lim AL , et al. Tumor hypoxia blocks Wnt processing and secretion through the induction of endoplasmic reticulum stress[J]. Mol Cell Biol, 2008,28(23):7212-7224. |
| [13] | 张翔云, 周华妙, 郭勇 . 黄连解毒汤等对乳腺癌荷瘤小鼠肿瘤酸性微环境pH值的影响[J]. 黑龙江中医药, 2016,45(4):64-65. |
| [14] | Flinck M, Kramer SH, Pedersen SF . Roles of pH in control of cell proliferation[J]. Acta Physiol (Oxf), 2018,223(3):e13068. |
| [15] | Peppicelli S, Andreucci E, Ruzzolini J . The acidic microenvironment as a possible niche of dormant tumor cells[J]. Cell Mol Life Sci, 2017,74(15):2761-2771. |
| [16] | Ryder C , McColl K,Zhong F, et al.Acidosis promotes Bcl-2 family-mediated evasion of apoptosis: Involvement of acid-sensing G protein-coupled receptor Gpr65 signaling to Mek/Erk[J]. J Biol Chem, 2012,287(33):27863-27867. |
| [17] | Dhup S, Dadhich RK, Porporato PE , et al. Multiple biological activities of lactic acid in cancer: Influences on tumor growth, angiogenesis and metastasis[J]. Curr Pharm Des, 2012,18(10):1319-1330. |
| [1] | Xiaodong CHAI,Ziwen SUN,Haishuang LI,Liangyi ZHU,Xiaodan LIU,Yantao LIU,Fei PEI,Qing CHANG. Clinicopathological characteristics of the CD8+ T lymphocytes infiltration and its mechanism in distinct molecular subtype of medulloblastoma [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 512-518. |
| [2] | Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU,Lu-lin MA. Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 781-792. |
| [3] | Jing LIU,Ai-dong LU,Ying-xi ZUO,Jun WU,Zhi-zhuo HUANG,Yue-ping JIA,Ming-ming DING,Le-ping ZHANG,Jiong QIN. Clinical characteristics and prognosis of seizures in 75 children with acute lymphoblastic leukemia [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 948-953. |
| [4] | GU Yang-chun,LIU Ying,XIE Chao,CAO Bao-shan. Pituitary immune-related adverse events induced by programmed cell death protein 1 inhibitors in advanced lung cancer patients: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 369-375. |
| [5] | LOU Xue,LIAO Li,LI Xing-jun,WANG Nan,LIU Shuang,CUI Ruo-mei,XU Jian. Methylation status and expression of TWEAK gene promoter region in peripheral blood of patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1020-1025. |
| [6] | GAO Ya-dong,ZHU An,LI Lu-di,ZHANG Tao,WANG Shuo,SHAN Dan-ping,LI Ying-zi,WANG Qi. Cytotoxicity and underlying mechanism of evodiamine in HepG2 cells [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1107-1114. |
| [7] | LIAO Xu-he,WANG Rong-fu,LIU Meng,CHEN Xue-qi,XIONG Yan,NONG Lin,YIN Lei,ZHANG Bing-ye,DU Yu-jing. Semiquantitative parameters of 18F-FDG PET/CT, gene mutation states of epidermal growth factor receptor and anaplastic lymphoma kinase in prognosis evaluation of patients with lung adenocarcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 246-254. |
| [8] | Lei-zhen SU,Jie CHEN,Xian LI,Ping JI. Effects of salinomycin on proliferation and apoptosis of oral squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 902-906. |
| [9] | Yi BAO,Juan-fen MO. Concordant point mutation of ETS-related gene (ERG) in tumor tissues from a synchronous multiple primary lung cancer: A case report [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 971-974. |
| [10] | Zhi-wei SUN,Jun JIA,Ying YANG,Chuan-ling LIU,Yan-jie XIAO,Jing YU,Xiao-dong ZHANG. Enteral nutrition support reduces toxicity of chemotherapy in patients with advanced or metastatic esophageal cancer [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 261-268. |
| [11] | Yi-xiang MA,Jing-wei LIU,Kang QI,Ji-xin ZHANG,Gang LIN,Hai-bo LIU,Xue-qian SHANG,Jian LI. Diagnosis and treatment of seven primary mediastinal yolk sac tumors [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1091-1095. |
| [12] | LI Man, LI Yuan, SUN Lin, SONG Jun-lai, LV Cong. High mobility group box 1 promotes apoptosis of astrocytes after oxygen glucose deprivation/reoxygenation by regulating the expression of Bcl-2 and Bax [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 785-791. |
| [13] | SUN Jing, SONG Wei-dong, YAN Si-yuan, XI Zhi-jun. Chloroquine inhibits viability of renal carcinoma cells and enhances sunitinib-induced caspase-dependent apoptosis [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 778-784. |
| [14] | WANG Hao, CHEN Liang, YE Xiao-yun. Triptolide induces oxidative stress and apoptosis and activates PIK3/Akt signaling pathway in TM4 sertoli cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 607-612. |
| [15] | MEI Fang, ZHAO Ting-ting, GAO Fei, ZHENG Jie. A rare pulmonary benign bi-phasic tumor: a case report of pulmonary adenofibroma and literature review [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 1076-1080. |
|
||