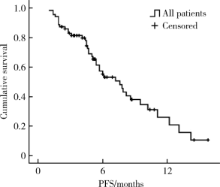
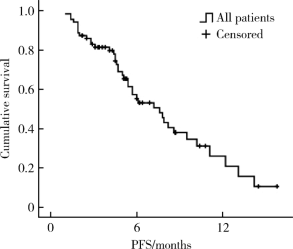
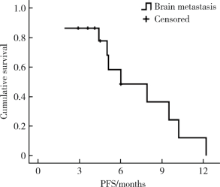
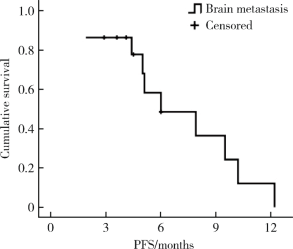
Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (2): 254-260. doi: 10.19723/j.issn.1671-167X.2020.02.010
Previous Articles Next Articles
Efficacy and safety of oral pyrotinib in HER2 positive metastatic breast cancer: real-world practice
Guo-hong SONG1,Hui-ping LI1,△( ),Li-jun DI1,Ying YAN1,Han-fang JIANG1,Ling XU2,Dong-gui WAN3,Ying LI4,Mo-pei WANG5,Yu XIAO5,Ru-yan ZHANG1,Ran RAN1,Huan WANG1
),Li-jun DI1,Ying YAN1,Han-fang JIANG1,Ling XU2,Dong-gui WAN3,Ying LI4,Mo-pei WANG5,Yu XIAO5,Ru-yan ZHANG1,Ran RAN1,Huan WANG1
- 1. Key Laboratory of Carcinogenesis and Translational Research, Ministry of Education; Department of Breast Oncology, Peking University Cancer Hospital & Institute, Beijing 100142, China
2. Breast Disease Center, Peking University First Hospital, Beijing 100034, China
3. Department of TCM Oncology, China-Japan Friendship Hospital, Beijing 100029, China
4. Department of Oncology, General Hospital of PLA, Beijing 100853, China
5. Department of Tumor Chemotherapy and Radiation Sickness, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R737.9
| [1] | Slamon DJ, Clark GM, Wong SG , et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene[J]. Science, 1987,235(4785):177-182. |
| [2] | Xu B, Hu X, Zheng H , et al. Outcomes of re-treatment with first-line trastuzumab plus a taxane in HER2 positive metastatic breast cancer patients after (neo) adjuvant trastuzumab: A prospective multicenter study[J]. Oncotarget, 2016,7(31):50643-50655. |
| [3] | Cobleigh M, Yardley D, Brufsky AM , et al. Baseline characteristics, treatment patterns, and outcomes in patients with HER2-positive metastatic breast cancer by hormone receptor status from SystHERs[J]. Clin Cancer Res, 2020,26(5):1105-1113. |
| [4] | Ma F, Li Q, Chen S , et al. Phase I study and biomarker analysis of pyrotinib, a novel irreversible pan-ErbB receptor tyrosine kinase inhibitor, in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer[J]. J Clin Oncol, 2017,35(27):3105-3112. |
| [5] | Li Q, Guan X, Chen S , et al. Safety, efficacy, and biomarker analysis of pyrotinib in combination with capecitabine in HER2-positive metastatic breast cancer patients: a phase Ⅰ clinical trial[J]. Clin Cancer Res, 2019,25(17):5212-5220. |
| [6] | Ma F, Ouyang Q, Li W , et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase Ⅱ study[J]. J Clin Oncol, 2019,37(29):2610-2619. |
| [7] | Jiang ZF, Yan M, Hu XC , et al. Pyrotinib combined with capecitabine in women with HER2+ metastatic breast cancer previously treated with trastuzumab and taxanes: A randomized phase Ⅲ study[J]. J Clin Oncol, 2019,37(15 suppl):1001. |
| [8] | Wong H, Leung R, Kwong A , et al. Integrating molecular mechanisms and clinical evidence in the management of trastuzumab resistant or refractory HER-2 + metastatic breast cancer [J]. Oncologist, 2011,16(11):1535-1546. |
| [9] | Dawood S, Broglio K, Buzdar AU , et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review[J]. J Clin Oncol, 2010,28(1):92-98. |
| [10] | Slamon DJ, Leyland-Jones B, Shak S , et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2[J]. N Engl J Med, 2001,344(11):783-792. |
| [11] | Burstein HJ, Keshaviah A, Baron AD , et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study[J]. Cancer, 2007,110(5):965-972. |
| [12] | Robert N, Leyland-Jones B, Asmar L , et al. Randomized phase Ⅲ study of trastuzumab, paclitaxel, and carboplatin compared with trastuzumab and paclitaxel in women with HER-2-overexpres-sing metastatic breast cancer[J]. J Clin Oncol, 2006,24(18):2786-2792. |
| [13] | Geyer CE, Forster J, Lindquist D , et al. Lapatinib plus capeci-tabine for HER2-positive advanced breast cancer[J]. N Engl J Med, 2006,355(26):2733-2743. |
| [14] | Cameron D, Casey M, Oliva C , et al. Lapatinib plus capecitabine in women with HER-2-positive advanced breast cancer: final survival analysis of a phase Ⅲ randomized trial[J]. Oncologist, 2010,15(9):924-934. |
| [15] | Xu BH, Jiang ZF, Chua D , et al. Lapatinib plus capecitabine in treating HER2-positive advanced breast cancer: efficacy, safety, and biomarker results from Chinese patients[J]. Chin J Cancer, 2011,30(5):327-335. |
| [16] | Baselga J, Cortés J, Kim SB , Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer[J]. N Engl J Med, 2012,366(2):109-119. |
| [17] | Verma S, Miles D, Gianni L , et al. Trastuzumab emtansine for HER2-positive advanced breast cancer[J]. N Engl J Med, 2012,367(19):1783-1791. |
| [18] | Leyland-Jones B . Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases[J]. J Clin Oncol, 2009,27(31):5278-5286. |
| [19] | Ramakrishna N, Temin S, Chandarlapaty S , et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: American Society of Clinical Oncology clinical practice guideline[J]. J Clin Oncol, 2014,32(19):2100-2108. |
| [20] | Ramakrishna N, Temin S, Chandarlapaty S , et al. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO clinical practice guideline update[J]. J Clin Oncol, 2018,36(27):2804-2807. |
| [21] | Bachelot T, Romieu G, Campone M , et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study[J]. Lancet Oncol, 2013,14(1):64-71. |
| [22] | Montemurro F, Ellis P, Delaloge S, et al. Safety and efficacy of trastuzumab emtansine(>T-DM1) in 399 patients with central nervous system metastases: Exploratory subgroup analysis from the KAMILLA study[J].Cancer Res, 2017, 77(4 Suppl):P1-12-10. |
| [1] | Ying TANG, Yongbo ZHANG, Danhong WU, Yanhong LIN, Fenghua LAN. Detection of pathogenic gene mutations in thirteen cases of congenital bilateral absence of vas deferens infertility patients [J]. Journal of Peking University (Health Sciences), 2024, 56(5): 763-774. |
| [2] | Jian-xun MA,You-chen XIA,Bi LI,Hong-mei ZHAO,Yu-tao LEI,Xi BU. Choice of immediate breast reconstructive methods after modified radical mastectomy [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 612-618. |
| [3] | Yun-jing ZHANG,Li-ying QIAO,Meng QI,Ying YAN,Wei-wei KANG,Guo-zhen LIU,Ming-yuan WANG,Yun-feng XI,Sheng-feng WANG. Development and validation of risk prediction model for new-onset cardiovascular diseases among breast cancer patients: Based on regional medical data of Inner Mongolia [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 471-479. |
| [4] | Xiao-juan ZHU,Hong ZHANG,Shuang ZHANG,Dong LI,Xin LI,Ling XU,Ting LI. Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 243-253. |
| [5] | Fang CHENG,Shao-ying YANG,Xing-xing FANG,Xuan WANG,Fu-tao ZHAO. Role of the CCL28-CCR10 pathway in monocyte migration in rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1074-1078. |
| [6] | Yue WANG,Shuang ZHANG,Hong ZHANG,Li LIANG,Ling XU,Yuan-jia CHENG,Xue-ning DUAN,Yin-hua LIU,Ting LI. Clinicopathological features and prognosis of hormone receptor-positive/human epidermal growth factor receptor 2-negative breast cancer [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 853-862. |
| [7] | Xiao-jing CHENG,Dong JIANG,Lian-hai ZHANG,Jiang-hua WANG,Ya-zhen LI,Jia-hui ZHAI,Bao-qi YAN,Lu-lu ZHANG,Xing-wang XIE,Zi-yu LI,Jia-fu JI. Preclinical study of T cell receptor specifically reactive with KRAS G12V mutation in the treatment of malignant tumors [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 884-895. |
| [8] | Zhi-qing LI,Bing YU,Ze-yu CAI,Ying-bao WANG,Xu ZHANG,Biao ZHOU,Xiao-hong FANG,Fang YU,Yi FU,Jin-peng SUN,Wei LI,Wei KONG. Naringenin inhibits thoracic aortic aneurysm formation in mice with Marfan syndrome [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 896-906. |
| [9] | Tian-yu CAI,Zhen-peng ZHU,Chun-ru XU,Xing JI,Tong-de LV,Zhen-ke GUO,Jian LIN. Expression and significance of fibroblast growth factor receptor 2 in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 628-635. |
| [10] | GU Yang-chun,LIU Ying,XIE Chao,CAO Bao-shan. Pituitary immune-related adverse events induced by programmed cell death protein 1 inhibitors in advanced lung cancer patients: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 369-375. |
| [11] | TIAN Jia-yi,ZHANG Xia,CHENG Gong,LIU Qing-hong,WANG Shi-yang,HE Jing. Serum interleukin-2 receptor α as a clinical biomarker in patients with systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1083-1087. |
| [12] | LIAO Xu-he,WANG Rong-fu,LIU Meng,CHEN Xue-qi,XIONG Yan,NONG Lin,YIN Lei,ZHANG Bing-ye,DU Yu-jing. Semiquantitative parameters of 18F-FDG PET/CT, gene mutation states of epidermal growth factor receptor and anaplastic lymphoma kinase in prognosis evaluation of patients with lung adenocarcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 246-254. |
| [13] | Xiao-peng ZHANG,Wei-yu ZHANG,Fei HUO,Hao HU,Qi WANG,Ke-xin XU. Outcome of surgical management and pathogenesis of female primary bladder neck obstruction [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1052-1055. |
| [14] | Xue-jun LIANG,Li-ying GONG,Fei ZHOU,De-min ZHOU,Jing-jing ZHU. Pharmacological effects of site specific conjugated anti-human epidermal growth factor receptor 2-antibody drug conjugate using unnatural amino acid technology [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 797-804. |
| [15] | Xin-yun YAO,Xiao-min GAO,Xiao-ying ZOU,Lin YUE. Role of endocytosis in cell surface CXC chemokine receptor 4 expression of stem cells from apical papilla [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 893-899. |
|
||