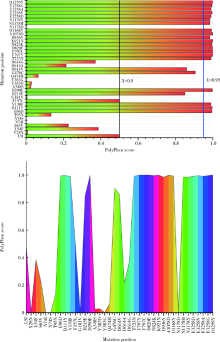
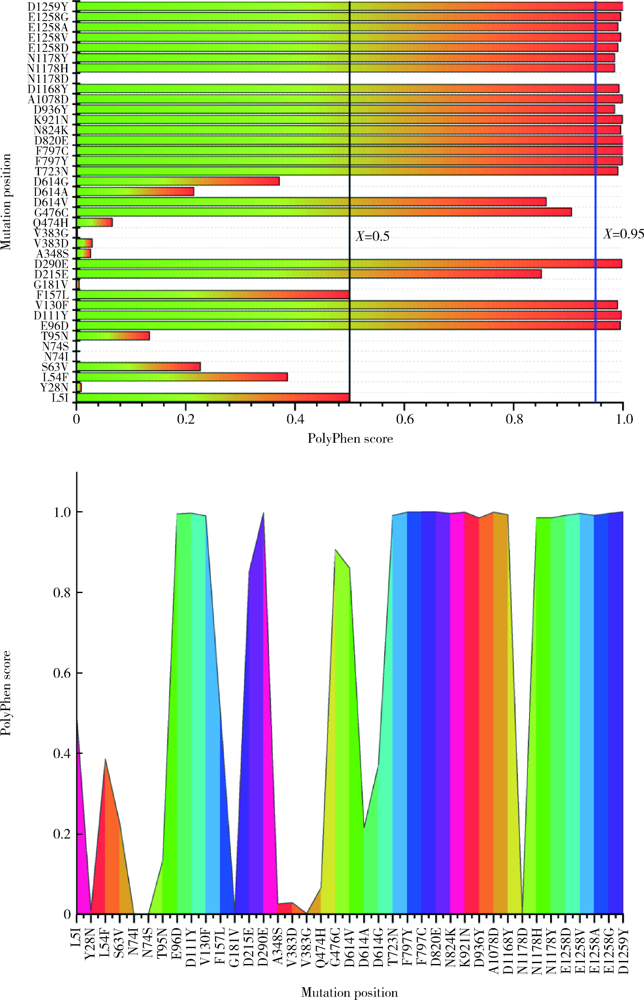
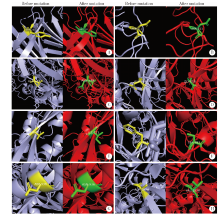
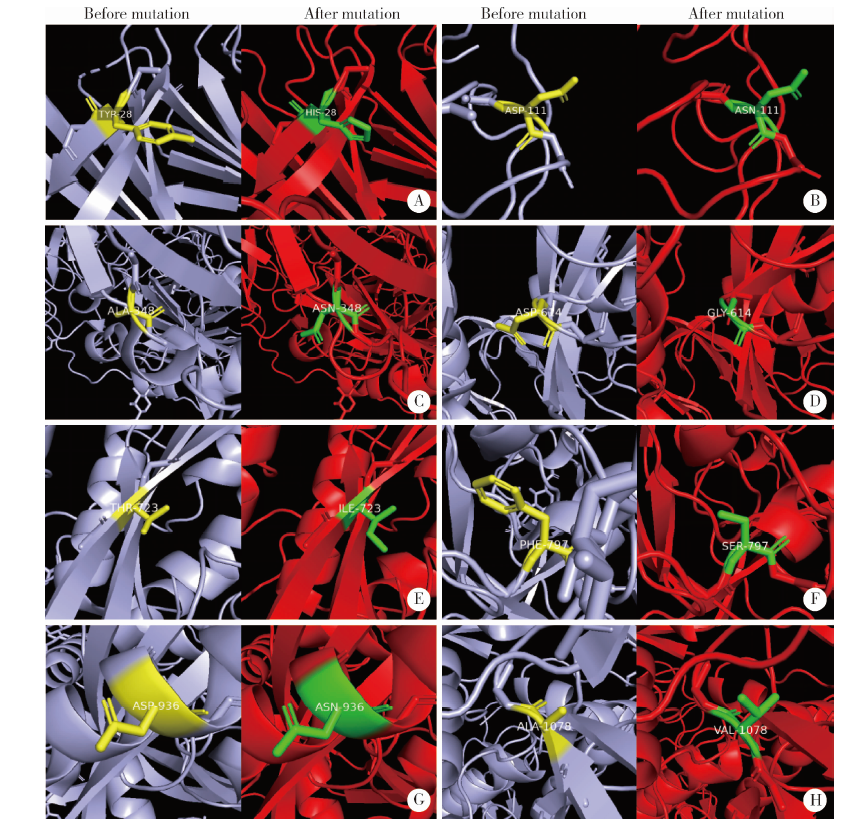
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (1): 150-158. doi: 10.19723/j.issn.1671-167X.2021.01.023
Previous Articles Next Articles
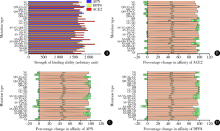
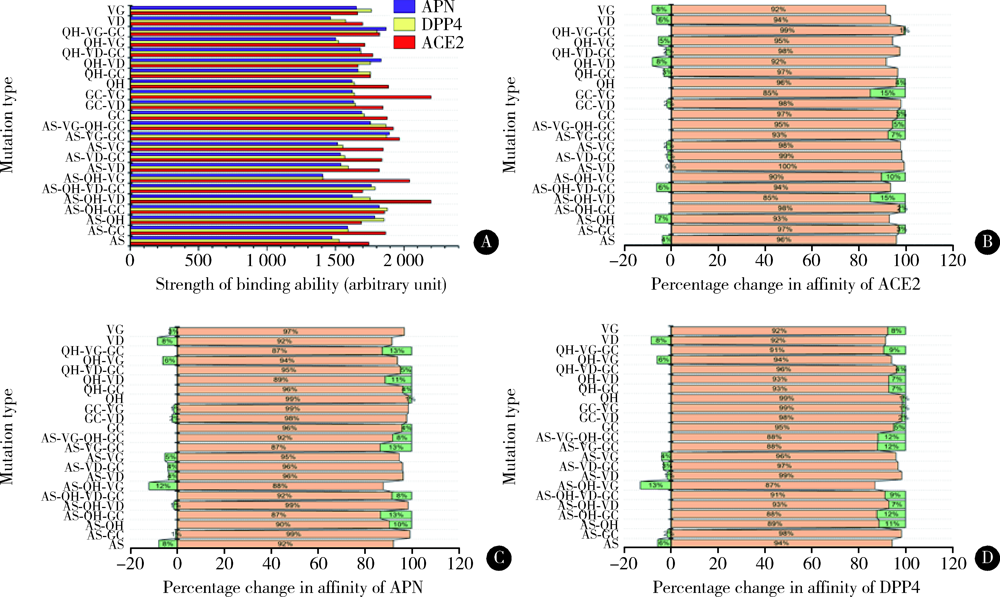
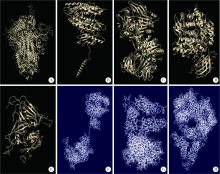
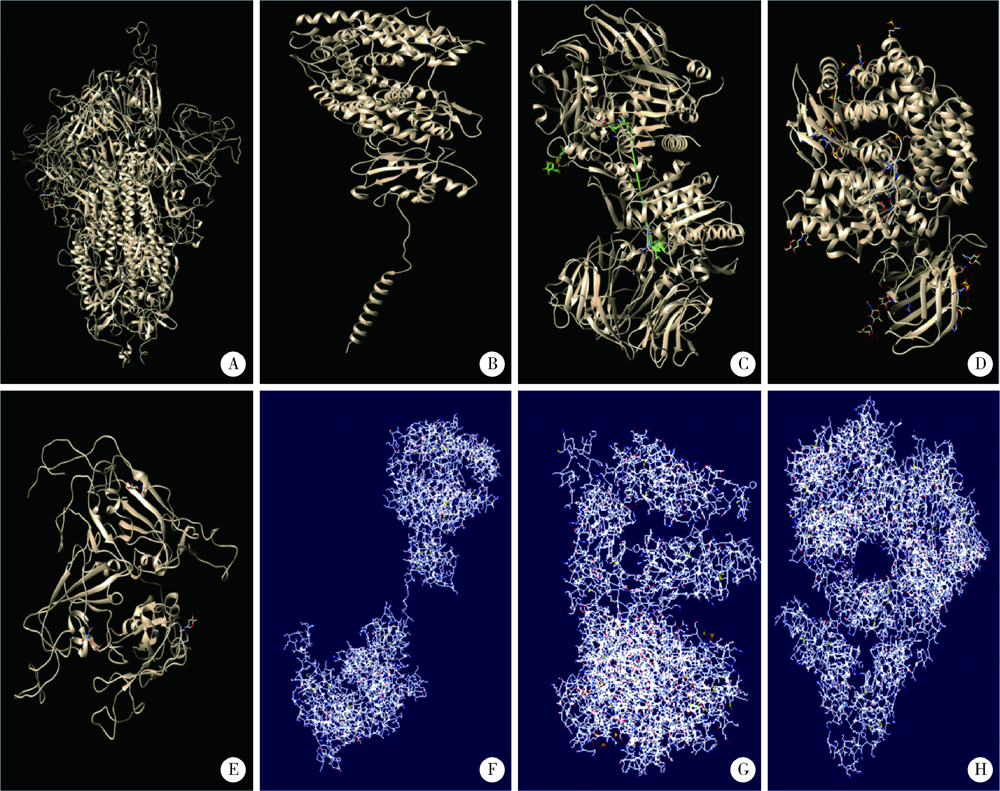
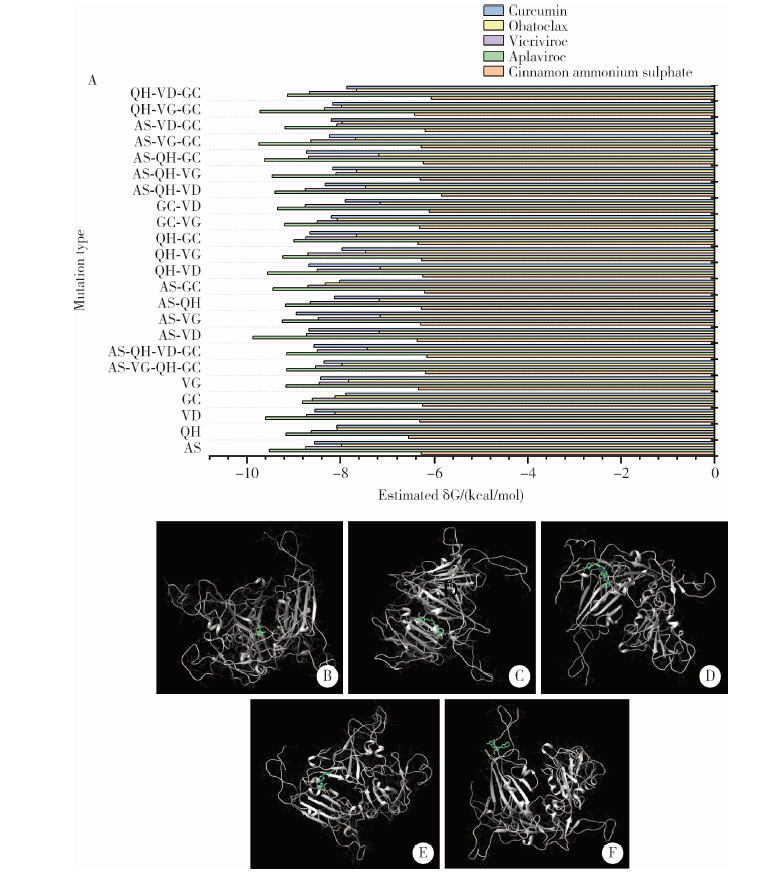
Homologous modeling and binding ability analysis of Spike protein after point mutation of severe acute respiratory syndrome coronavirus 2 to receptor proteins and potential antiviral drugs
CAO Ze,WANG Le-tong,LIU Zhen-ming( )
)
- State Key Laboratory of Natural and Biomimetic Drugs, Peking University School of Pharmaceutical Sciences, Beijing 100191, China
CLC Number:
- R373.19
| [1] |
Liu J, Zheng X, Tong Q, et al. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human patho-genic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV[J]. J Med Virol, 2020,92(5):491-494.
doi: 10.1002/jmv.25709 pmid: 32056249 |
| [2] | Wang C, Liu Z, Chen Z, et al. The establishment of reference sequence for SARS-CoV-2 and variation analysis[J]. J Med Virol, 2020,10(1002):25762. |
| [3] |
Ma Y, Wu L, Shaw N, et al. Structural basis and functional analysis of the SARS coronavirus nsp14-nsp10 complex[J]. Proc Natl Acad Sci USA, 2015,112(30):9436-9441.
doi: 10.1073/pnas.1508686112 pmid: 26159422 |
| [4] |
Kim D, Lee JY, Yang JS, et al. The architecture of SARS-CoV-2 transcriptome[J]. Cell, 2020,181(4):914-921.
doi: 10.1016/j.cell.2020.04.011 pmid: 32330414 |
| [5] |
Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding[J]. Lancet, 2020,395(10224):565-574.
doi: 10.1016/S0140-6736(20)30251-8 pmid: 32007145 |
| [6] |
Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin[J]. Nature, 2020,579(7798):270-273.
doi: 10.1038/s41586-020-2012-7 pmid: 32015507 |
| [7] |
Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2[J]. Science, 2020,367(6485):1444-1448.
doi: 10.1126/science.abb2762 pmid: 32132184 |
| [8] |
Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 Spike glycoprotein[J]. Cell, 2020,181(2):281-292.
doi: 10.1016/j.cell.2020.02.058 pmid: 32155444 |
| [9] |
Chan CM, Chu H, Wang Y, et al. Carcinoembryonic antigen-related cell adhesion molecule 5 is an important surface attachment factor that facilitates entry of Middle East respiratory syndrome coronavirus[J]. J Virol, 2016,90(20):9114-9127.
doi: 10.1128/JVI.01133-16 pmid: 27489282 |
| [10] |
Cui J, Li F, Shi ZL. Origin and evolution of pathogenic corona-viruses[J]. Nat Rev Microbiol, 2019,17(3):181-192.
doi: 10.1038/s41579-018-0118-9 pmid: 30531947 |
| [11] |
Chu H, Chan CM, Zhang X, et al. Middle East respiratory syndrome coronavirus and bat coronavirus HKU9 both can utilize GRP78 for attachment onto host cells[J]. J Biol Chem, 2018,293(30):11709-11726.
doi: 10.1074/jbc.RA118.001897 pmid: 29887526 |
| [12] |
Satija N, Lal SK. The molecular biology of SARS coronavirus[J]. Ann N Y Acad Sci, 2007,1102(1):26-38.
doi: 10.1196/annals.1408.002 |
| [13] |
Chen L, Gui C, Luo X, et al. Cinanserin is an inhibitor of the 3C-like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro[J]. J Virol, 2005,79(11):7095-7103.
doi: 10.1128/JVI.79.11.7095-7103.2005 pmid: 15890949 |
| [14] |
Emmelkamp JM, Rockstroh JK. CCR5 antagonists: comparison of efficacy, side effects, pharmacokinetics and interactions: review of the literature[J]. Eur J Med Res, 2007,12(9):409-417.
pmid: 17933722 |
| [15] |
Lagadinou ED, Sach A, Callahan K, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells[J]. Cell Stem Cell, 2013,12(3):329-341.
doi: 10.1016/j.stem.2012.12.013 |
| [16] |
Andersen PI, Krpina K, Ianevski A, et al. Novel antiviral activities of obatoclax, emetine, niclosamide, brequinar, and homoharringtonine[J]. Viruses, 2019,11(10):964.
doi: 10.3390/v11100964 |
| [17] |
Wen CC, Kuo YH, Jan JT, et al. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus[J]. J Med Chem, 2007,50(17):4087-4095.
doi: 10.1021/jm070295s pmid: 17663539 |
| [18] |
Aliyari Serej Z, Ebrahimi Kalan A, Mehdipour A, et al. Regulation and roles of CD26/DPPIV in hematopoiesis and diseases[J]. Biomed Pharmacother, 2017,91:88-94.
doi: 10.1016/j.biopha.2017.04.074 pmid: 28448874 |
| [19] |
Cheng F, Yuan G, He J, et al. Dysregulation of DPP4 is associa-ted with the AMPK/JAK2/STAT3 pathway in adipocytes under insulin resistance status and liraglutide intervention[J]. Diabetes Metab Syndr Obes, 2019,12:2635-2644.
doi: 10.2147/DMSO.S229838 pmid: 31849507 |
| [20] |
Song Z, Xu Y, Bao L, et al. From SARS to MERS, thrusting coronaviruses into the spotlight[J]. Viruses, 2019,11(1):59.
doi: 10.3390/v11010059 |
| [21] |
Li Y, Zhang Z, Yang L, et al. The MERS-CoV receptor DPP4 as a candidate binding target of the SARS-CoV-2 Spike[J]. iScience, 2020,23(6):101160.
doi: 10.1016/j.isci.2020.101160 pmid: 32405622 |
| [22] |
Nichols WG, Steel HM, Bonny T, et al. Hepatotoxicity observed in clinical trials of aplaviroc[J]. Antimicrob Agents Chemother, 2008,52(3):858-865.
doi: 10.1128/AAC.00821-07 pmid: 18070967 |
| [23] |
Kitrinos KM, Amrine-Madsen H, Irlbeck DM, et al. Virologic failure in therapy-naive subjects on aplaviroc plus lopinavir-ritonavir: detection of aplaviroc resistance requires clonal analysis of envelope[J]. Antimicrob Agents Chemother, 2009,53(3):1124-1131.
doi: 10.1128/AAC.01057-08 pmid: 19075068 |
| [1] | Jian-bin LI,Meng-na LYU,Qiang CHI,Yi-lin PENG,Peng-cheng LIU,Rui WU. Early prediction of severe COVID-19 in patients with Sjögren’s syndrome [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1007-1012. |
| [2] | Yun-fei SHI,Hao-jie WANG,Wei-ping LIU,Lan MI,Meng-ping LONG,Yan-fei LIU,Yu-mei LAI,Li-xin ZHOU,Xin-ting DIAO,Xiang-hong LI. Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 521-529. |
| [3] | Yan XIONG,Bo ZHANG,Li-gong NIE,Shi-kai WU,Hu ZHAO,Dong LI,Ji-ting DI. Thoracic SMARCA4-deficient undifferentiated tumor-pathological diagnosis and combined immune checkpoint inhibitor treatment [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 351-356. |
| [4] | Qiu-jun ZHOU,Pan GONG,Xian-ru JIAO,Zhi-xian YANG. Clinical and molecular genetic analysis of Angelman syndrome with oculocutaneous albinism type 2: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 181-185. |
| [5] | Xiao-jing CHENG,Dong JIANG,Lian-hai ZHANG,Jiang-hua WANG,Ya-zhen LI,Jia-hui ZHAI,Bao-qi YAN,Lu-lu ZHANG,Xing-wang XIE,Zi-yu LI,Jia-fu JI. Preclinical study of T cell receptor specifically reactive with KRAS G12V mutation in the treatment of malignant tumors [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 884-895. |
| [6] | Zhan-peng SHANG,Yang YI,Rong YU,Jing-jing FAN,Yu-xi HUANG,Xue QIAO,Min YE. Bioactive compounds of Jingfang Granules against SARS-CoV-2 virus proteases 3CLpro and PLpro [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 907-919. |
| [7] | Cai-peng QIN,Yu-xuan SONG,Meng-ting DING,Fei WANG,Jia-xing LIN,Wen-bo YANG,Yi-qing DU,Qing LI,Shi-jun LIU,Tao XU. Establishment of a mutation prediction model for evaluating the efficacy of immunotherapy in renal carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 663-668. |
| [8] | Xi CHEN,Si-yue WANG,En-ci XUE,Xue-heng WANG,He-xiang PENG,Meng FAN,Meng-ying WANG,Yi-qun WU,Xue-ying QIN,Jing LI,Tao WU,Hong-ping ZHU,Jing LI,Zhi-bo ZHOU,Da-fang CHEN,Yong-hua HU. Exploring the association between de novo mutations and non-syndromic cleft lip with or without palate based on whole exome sequencing of case-parent trios [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 387-393. |
| [9] | FENG Ke,NI Jing-jing,XIA Yan-qing,QU Xiao-wei,ZHANG Hui-juan,WAN Feng,HONG Kai,ZHANG Cui-lian,GUO Hai-bin. Genetic analysis of three cases of acephalic spermatozoa syndrome caused by SUN5 mutation and the outcome of assisted reproductive technology [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 803-807. |
| [10] | YANG Lin-cheng,ZHANG Rui-tao,GUO Li-jun,XIAO Han,ZU Ling-yun,ZHANG You-yi,CHENG Qin,ZHAO Zhi-ling,GE Qing-gang,GAO Wei. Hypoxia and inflammation are risk factors for acute myocardial injury in patients with coronavirus disease 2019 [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 159-166. |
| [11] | WU Jun-yi,YU Miao,SUN Shi-chen,FAN Zhuang-zhuang,ZHENG Jing-lei,ZHANG Liu-tao,FENG Hai-lan,LIU Yang,HAN Dong. Detection of EDA gene mutation and phenotypic analysis in patients with hypohidrotic ectodermal dysplasia [J]. Journal of Peking University (Health Sciences), 2021, 53(1): 24-33. |
| [12] | Fang BAO,Wei-li SHI,Jing HU,Di ZHANG,Dong-han GAO,Yun-xia XIA,Hong-mei JING,Xiao-yan KE,Qing-gang GE,Ning SHEN. Analysis of the correlation between lymphocyte subsets and severity of corona virus disease 19 [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1075-1081. |
| [13] | Yi BAO,Juan-fen MO. Concordant point mutation of ETS-related gene (ERG) in tumor tissues from a synchronous multiple primary lung cancer: A case report [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 971-974. |
| [14] | Xiao-ning YUAN,Qing-yang MENG,Ning SHEN,Yu-xuan LI,Chao LIANG,Man CUI,Qing-gang GE,Xiao-guang LI,Kun TAN,Qian CHEN,Jing WANG,Xiao-yong ZENG. Detection and evaluation of SARS-CoV-2 nucleic acid contamination in corona virus disease 19 ward surroundings and the surface of medical staff’s protective equipment [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 803-808. |
| [15] | Qiu WANG,Jin-yu GUO,Hong SUN,Ling WANG,Ju-su YING,Hui-xin LIU. Investigation of protective exposure risk events in nurses against corona virus disease 2019 in Wuhan [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 711-714. |
|
||