Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (1): 40-45. doi: 10.19723/j.issn.1671-167X.2021.01.007
Previous Articles Next Articles
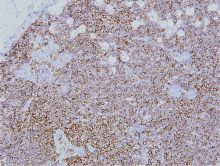
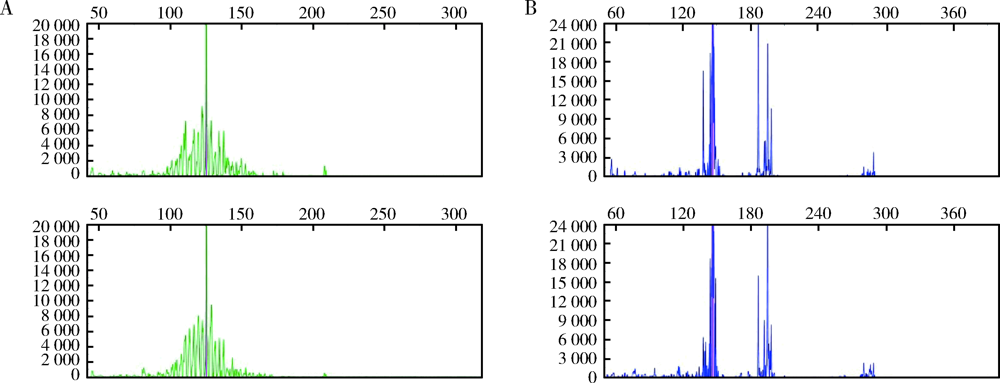
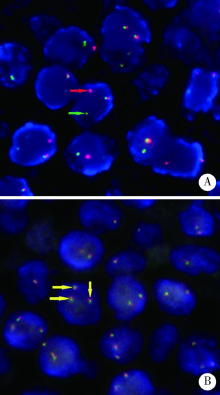
Clinicopathological analysis of mucosa associated lymphoid tissue lymphoma secondary to Sjögren’s syndrome in salivary gland
CHI Yan-ting1,2,ZHANG Yan-ping3,ZHANG Qiu-lu4,LIU Cui-ling5,6,Δ( ),LI bin-bin1,2,Δ(
),LI bin-bin1,2,Δ( )
)
- 1. Department of Oral Pathology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. Research Unit of Precision Pathologic Diagnosis in Tumors of the Oral and Maxillofacial Regions, Chinese Academy of Medical Sciences (2019RU034), Beijing 100081, China
3. Department of Pathology, First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, China
4. West China School of Medicine, Sichuan University, Chengdu 610041, China
5. Department of Pathology, Peking University School of Basic Medical Science, Beijing 100191, China
6. Department of Pathology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R733.41
| [1] |
Isaacson PG, Wright DH. Malignant lymphoma of mucosa associate lymphoid tissue: A distinctive type of B cell lymphoma[J]. Cancer, 1983,52(8):1410-1416.
doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3 pmid: 6193858 |
| [2] | Isaacson PG, Chott A, Nakamura S, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) [M]//WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: LARC Press, 2008: 214-217. |
| [3] |
Cohen SM, Petryk M, Varma M, et al. Non-Hodgkin’s lymphoma of mucosa-associated lymphoid tissue[J]. Oncologist, 2006,11(10):1100-1117.
doi: 10.1634/theoncologist.11-10-1100 pmid: 17110630 |
| [4] |
Hirokawa Y, Fujikawa R, Arai Y, et al. Primary thymic MALT lymphoma in a patient with Sjögren’s syndrome and multiple lung cysts: A case report[J]. Surg Case Rep, 2019,5(1):138.
doi: 10.1186/s40792-019-0696-4 pmid: 31478101 |
| [5] |
Titsinides S, Nikitakis N, Piperi E, et al. MALT lymphoma of minor salivary glands in a Sjögren’s syndrome patient: A case report and review of literature[J]. J Oral Maxillofac Res, 2017,8(1):e5.
doi: 10.5037/jomr.2017.8105 pmid: 28496965 |
| [6] |
Jackson AE, Mian M, Kalpadakis C, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue of the salivary glands: A multicenter, international experience of 248 patients (IELSG 41)[J]. Oncologist, 2015,20(10):1149-1153.
doi: 10.1634/theoncologist.2015-0180 pmid: 26268740 |
| [7] |
De Vita S, Gandolfo S. Predicting lymphoma development in patients with Sjögren’s syndrome[J]. Expert Rev Clin Immunol, 2019,15(9):929-938.
doi: 10.1080/1744666X.2019.1649596 pmid: 31347413 |
| [8] |
Vitali C, Bombardieri S, Jnnsson R, et al. Classification criteria for Sjögren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group[J]. Ann Rheum Dis, 2002,61(6):554-558.
doi: 10.1136/ard.61.6.554 pmid: 12006334 |
| [9] |
A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The non-Hodgkin’s lymphoma classification project[J]. Blood, 1997,89(11):3909-3918.
pmid: 9166827 |
| [10] |
Spencer J, Diss T, Isaacson PG. A study of the properties of a low-grade mucosal B-cell lymphoma using a monoclonal antibody specific for the tumour immunoglobulin[J]. J Pathol, 1990,160(3):231-238.
doi: 10.1002/path.1711600309 pmid: 2335804 |
| [11] | 徐卫. 小B细胞淋巴瘤的诊断[J]. 临床内科杂志, 2015,32(3):163-165. |
| [12] | 刘霜竹, 张艳青, 郁多男, 等. 转录因子Pax5 可不依赖与启动子结合促进B细胞淋巴瘤生长[J]. 中国实验血液学杂志, 2017,25(6):1652-1657. |
| [13] |
Xue K, Song JZ, Yang Y, et al. PAX5 promotes pre-B cell proli-feration by regulating the expressionof pre-B cell receptor and its downstream signaling[J]. Mol Immunol, 2016,73:1-9.
doi: 10.1016/j.molimm.2016.03.007 pmid: 27016671 |
| [14] | 王红霞, 陈昊, 林海月, 等. MALToma Ig重链蛋白、IgH基因重排/断裂检测的临床意义[J]. 临床与实验病理学杂志, 2019,35(8):902-906. |
| [15] |
Weiss LM, Spagnolo DV. Assessment of clonality in lymphoid proliferation[J]. Am J Pathol, 1993,142(6):1679-1682.
pmid: 8506940 |
| [16] |
Chen YL, Su IJ, Cheng HY, et al. BIOMED-2 protocols to detect clonal immunoglobulin and T-cell receptor gene rearrangements in B- and T-cell lymphomas in southern Taiwan[J]. Leuk Lymphoma, 2010,51(4):650-655.
doi: 10.3109/10428191003660631 pmid: 20233058 |
| [17] |
HalldÓrsdÓttir AM, Zehnbauer BA, Burack WR. Application of BIOMED-2 clonality assays to formalin-fixed paraffin embedded follicular lymphoma specimens: Superior performance of the IGK assays compared to IGH for suboptimal specimens[J]. Leuk Lymphoma, 2007,48(7):1338-1343.
doi: 10.1080/10428190701377022 pmid: 17613763 |
| [18] |
Du MQ. MALT lymphoma: Genetic abnormalities, immunological stimulation and molecular mechanism[J]. Best Pract Res Clin Haematol, 2017,30(1-2):13-23.
doi: 10.1016/j.beha.2016.09.002 pmid: 28288707 |
| [19] |
Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma)[J]. CA Cancer J Clin, 2016,66(2):153-171.
doi: 10.3322/caac.21330 pmid: 26773441 |
| [20] | Ye H, Reinstein ED, Bacon CM, et al. Chromosomal translocations involving BCL6 in MALT lymphoma[J]. Haematologlca, 2008,93(1):145-146. |
| [21] |
何晨, 毛峥嵘. FISH 技术在淋巴瘤分子特征中的研究和应用进展[J]. 中国肿瘤临床, 2013,40(10):608-611.
doi: doi:10.3969/j.issn. 1000-8179.2013.10.014 |
| [22] | 林海月, 王红霞, 陈昊. 黏膜相关淋巴组织淋巴瘤诊断进展[J]. 临床与实验病理学杂志, 2017,33(9):1010-1012. |
| [1] | Yu-xiao WU,Yi-fan KANG,Qian-ying MAO,Zi-meng LI,Xiao-feng SHAN,Zhi-gang CAI. Application of methylene blue near-infrared fluorescence imaging for oral sentinel lymph node mapping in rats [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 684-688. |
| [2] | Wei WANG,Xin LI,Ping LIU,Ying DONG. Clinical value of fluorescence in situ hybridization with MDM2 and DDIT3 probe in diagnosis of liposarcoma [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 228-233. |
| [3] | YU Yan-fei,HE Shi-ming,WU Yu-cai,XIONG Sheng-wei,SHEN Qi,LI Yan-yan,YANG Feng,HE Qun,LI Xue-song. Clinicopathological features and prognosis of fumarate hydratase deficient renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 640-646. |
| [4] | Bing-wei HUANG,Jie WANG,Peng ZHANG,Zhe LI,Si-cheng BI,Qiang WANG,Cai-bo YUE,Kun-lin YANG,Xue-song LI,Li-qun ZHOU. Application of indocyanine green in complex upper urinary tract repair surgery [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 651-656. |
| [5] | Shu-dong ZHANG,Peng HONG,Bin-shuai WANG,Shao-hui DENG,Fan ZHANG,Li-yuan TAO,Cai-guang CAO,Zhen-hua HU,Lu-lin MA. Usefulness of the indocyanine green fluorescence imaging technique in laparoscopic partial nephrectomy [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 657-662. |
| [6] | Ru MA,Xin-bao LI,Feng-cai YAN,Yu-lin LIN,Yan LI. Clinical evaluation of tumor-stroma ratio in pseudomyxoma peritonei from the appendix [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 240-246. |
| [7] | Xiao-ran NING,Zi-qiao WANG,Shan-shan ZHANG,Xia ZHANG,Su-mei TANG,Yan-ying LIU. Application of ultrasonography scoring system in the assessment of IgG4-related sialadenitis [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1032-1035. |
| [8] | Xiao-peng ZHANG,Wei-yu ZHANG,Fei HUO,Hao HU,Qi WANG,Ke-xin XU. Outcome of surgical management and pathogenesis of female primary bladder neck obstruction [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1052-1055. |
| [9] | Jia-yu WANG,Mei-ping ZHAO. Fluorescence assay for the detection of apurinic/apyrimidinic endonuclease 1 (APE1) activity in human blood samples [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 487-492. |
| [10] | Chun-feng ZHANG,Yun LIU,Min LU,Xiao-juan DU. Expression of hUTP14a in non-small cell lung cancer [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 145-150. |
| [11] | Lei LIU,Li-hua WANG,Yu-bo REN,Xiao-song RAO,Shao-min YANG. Clinicopathological analysis of aggressive angiomyxoma of soft tissue in abdomino-pelvic cavity [J]. Journal of Peking University(Health Sciences), 2018, 50(6): 1098-1101. |
| [12] | MEI Fang, ZHAO Ting-ting, GAO Fei, ZHENG Jie. A rare pulmonary benign bi-phasic tumor: a case report of pulmonary adenofibroma and literature review [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 1076-1080. |
| [13] | FENG Yong-liang, FAN Jing-hui, LIN Xian-juan, YANG Ji-chun, CUI Qing-hua, TANG Xin-jing, XU Guo-heng, GENG Bin. Facilitating the measurement of circulatory hydrogen sulfide with fluorescence probe-coated microplates [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 1060-1065. |
| [14] | LIU Chang, CUI Li-gang, WANG Hong-lei. Renal Ewing’s sarcoma/primitive neuroectodermal tumor: a case report and literature review [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 919-923. |
| [15] | WANG Fang, ZHANG Yan-qin, DING Jie, YU Li-xia. Detection of large deletions in X linked Alport syndrome using competitive multiplex fluorescence polymerase chain reaction [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 760-767. |
|
||