Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (1): 124-132. doi: 10.19723/j.issn.1671-167X.2023.01.019
Previous Articles Next Articles
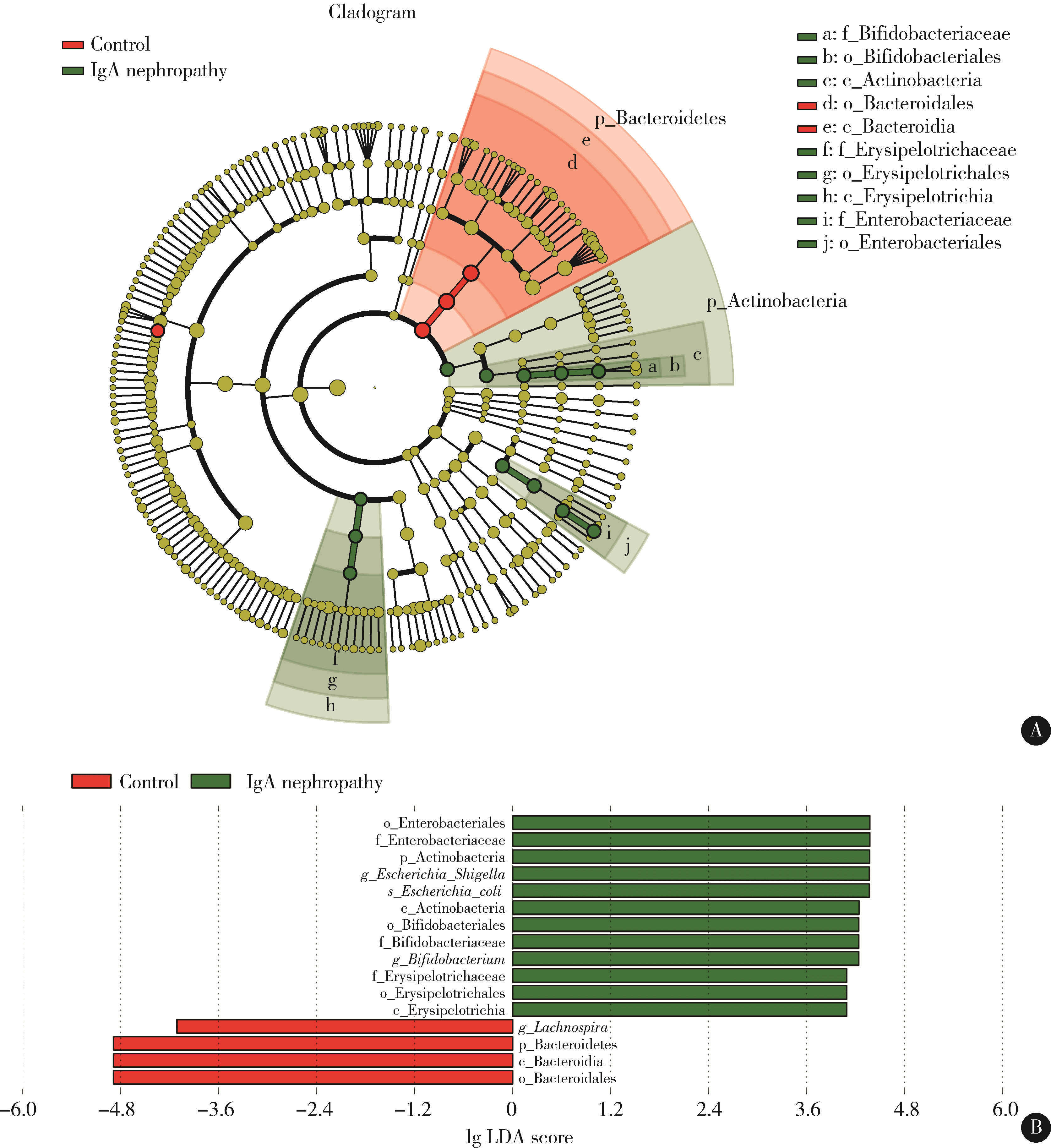
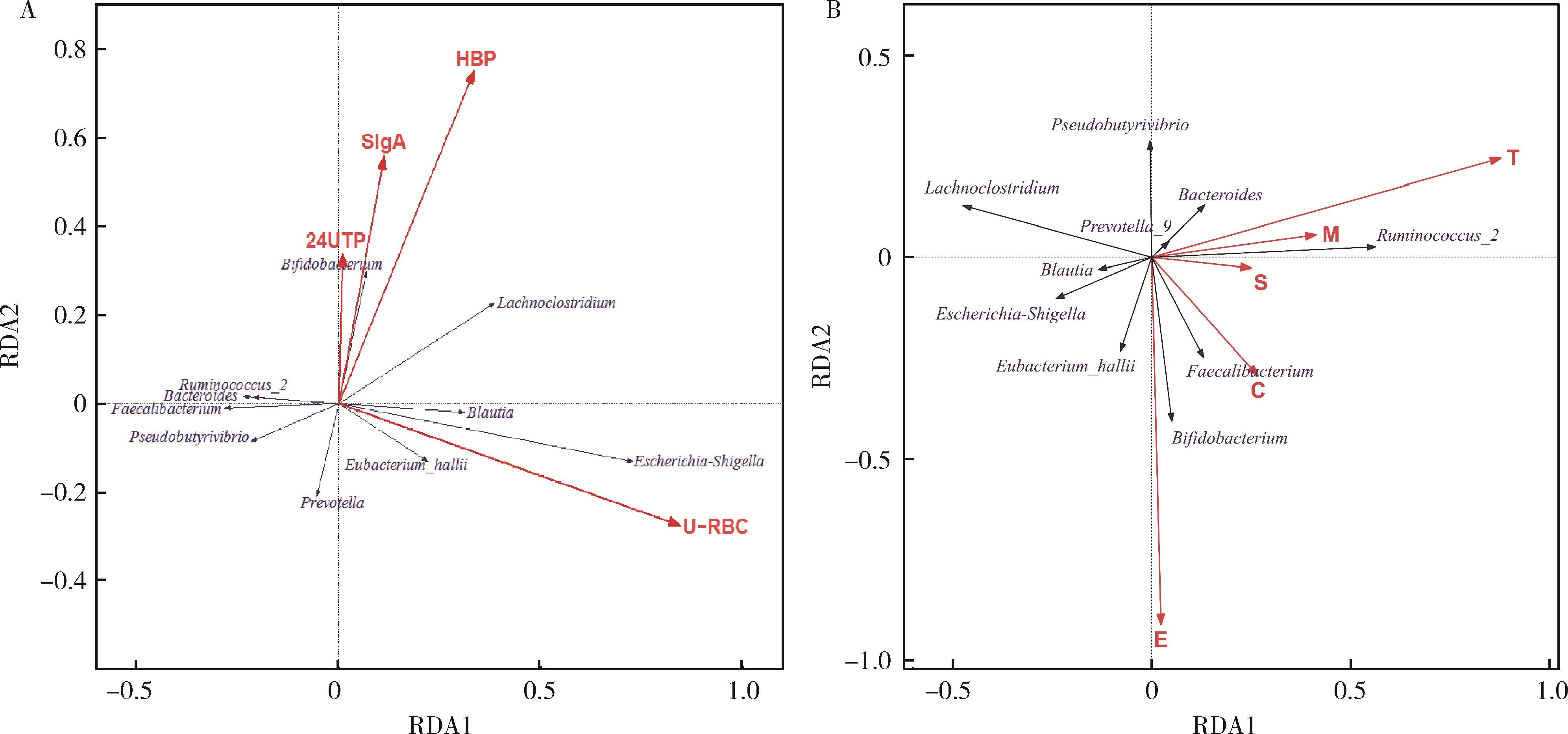
Changes of gut microflora in newly diagnosed IgA nephropathy patients and its correlation with clinical risk factors
- Department of Nephrology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R692.3
| 1 |
Schena FP , Nistor I . Epidemiology of IgA nephropathy: A global perspective[J]. Semin Nephrol, 2018, 38 (5): 435- 442.
doi: 10.1016/j.semnephrol.2018.05.013 |
| 2 |
Wang M , Lv J , Zhang X , et al. Secondary IgA nephropathy shares the same immune features with primary IgA nephropathy[J]. Kidney Int Rep, 2020, 5 (2): 165- 172.
doi: 10.1016/j.ekir.2019.10.012 |
| 3 |
Hu X , Du J , Xie Y , et al. Fecal microbiota characteristics of Chinese patients with primary IgA nephropathy: A cross-sectional study[J]. BMC Nephrol, 2020, 21 (1): 97.
doi: 10.1186/s12882-020-01741-9 |
| 4 |
De Angelis M , Montemurno E , Piccolo M , et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN)[J]. PLoS One, 2014, 9 (6): e99006.
doi: 10.1371/journal.pone.0099006 |
| 5 |
Hobby GP , Karaduta O , Dusio GF , et al. Chronic kidney disease and the gut microbiome[J]. Am J Physiol Renal Physiol, 2019, 316 (6): F1211- F1217.
doi: 10.1152/ajprenal.00298.2018 |
| 6 |
Munyaka PM , Eissa N , Bernstein CN , et al. Antepartum antibio-tic treatment increases offspring susceptibility to experimental colitis: A role of the gut microbiota[J]. PLoS One, 2015, 10 (11): e0142536.
doi: 10.1371/journal.pone.0142536 |
| 7 |
Edgar RC . UPARSE: highly accurate OTU sequences from microbial amplicon reads[J]. Nat Methods, 2013, 10 (10): 996- 998.
doi: 10.1038/nmeth.2604 |
| 8 | Cole JR , Wang Q , Cardenas E , et al. The ribosomal database project: Improved alignments and new tools for rRNA analysis[J]. Nucleic Acids Res, 2009, 37 (Suppl 1): D141- D145. |
| 9 |
Segata N , Izard J , Waldron L , et al. Metagenomic biomarker discovery and explanation[J]. Genome Biol, 2011, 12 (6): R60.
doi: 10.1186/gb-2011-12-6-r60 |
| 10 |
Mitchell RJ , Hewison RL , Fielding DA , et al. Decline in atmospheric sulphur deposition and changes in climate are the major drivers of long-term change in grassland plant communities in Scotland[J]. Environ Pollut, 2018, 235, 956- 964.
doi: 10.1016/j.envpol.2017.12.086 |
| 11 | Li H , Li T , Beasley DE , et al. Diet diversity is associated with beta but not alpha diversity of pika gut microbiota[J]. Front Microbiol, 2016, 7, 1169. |
| 12 |
Suzuki H , Kiryluk K , Novak J , et al. The pathophysiology of IgA nephropathy[J]. J Am Soc Nephrol, 2011, 22 (10): 1795- 1803.
doi: 10.1681/ASN.2011050464 |
| 13 |
Nakajima A , Vogelzang A , Maruya M , et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria[J]. J Exp Med, 2018, 215 (8): 2019- 2034.
doi: 10.1084/jem.20180427 |
| 14 |
Floege J , Feehally J . The mucosa-kidney axis in IgA nephropathy[J]. Nat Rev Nephrol, 2016, 12 (3): 147- 156.
doi: 10.1038/nrneph.2015.208 |
| 15 |
Olive C , Allen AC , Harper SJ , et al. Expression of the mucosal T cell receptor V region repertoire in patients with IgA nephropathy[J]. Kidney Int, 1997, 52 (4): 1047- 1053.
doi: 10.1038/ki.1997.427 |
| 16 |
McCarthy DD , Kujawa J , Wilson C , et al. Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy[J]. J Clin Invest, 2011, 121 (10): 3991- 4002.
doi: 10.1172/JCI45563 |
| 17 |
Kiryluk K , Li Y , Scolari F , et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens[J]. Nat Genet, 2014, 46 (11): 1187- 1196.
doi: 10.1038/ng.3118 |
| 18 |
Grosserichter-Wagener C , Radjabzadeh D , van der Weide H , et al. Differences in systemic IgA reactivity and circulating Th subsets in healthy volunteers with specific microbiota enterotypes[J]. Front Immunol, 2019, 10, 341.
doi: 10.3389/fimmu.2019.00341 |
| 19 |
Barko PC , McMichael MA , Swanson KS , et al. The gastrointestinal microbiome: A review[J]. J Vet Intern Med, 2018, 32 (1): 9- 25.
doi: 10.1111/jvim.14875 |
| 20 |
Gomaa EZ . Human gut microbiota/microbiome in health and diseases: A review[J]. Antonie Van Leeuwenhoek, 2020, 113 (12): 2019- 2040.
doi: 10.1007/s10482-020-01474-7 |
| 21 |
包文晗, 王悦. 慢性肾脏病患者肠道菌群的变化及影响研究[J]. 中国全科医学, 2018, 21 (24): 2927- 2931.
doi: 10.3969/j.issn.1007-9572.2018.00.168 |
| 22 |
Gibiino G , Lopetuso LR , Scaldaferri F , et al. Exploring Bacteroidetes: Metabolic key points and immunological tricks of our gut commensals[J]. Dig Liver Dis, 2018, 50 (7): 635- 639.
doi: 10.1016/j.dld.2018.03.016 |
| 23 |
Lo Presti A , Zorzi F , Del Chierico F , et al. Fecal and mucosal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease[J]. Front Microbiol, 2019, 10, 1655.
doi: 10.3389/fmicb.2019.01655 |
| 24 |
Amabebe E , Robert FO , Agbalalah T , et al. Microbial dysbiosis-induced obesity: Role of gut microbiota in homoeostasis of energy metabolism[J]. Br J Nutr, 2020, 123 (10): 1127- 1137.
doi: 10.1017/S0007114520000380 |
| 25 |
Coppo R . The gut-kidney axis in IgA nephropathy: Role of microbiota and diet on genetic predisposition[J]. Pediatr Nephrol, 2018, 33 (1): 53- 61.
doi: 10.1007/s00467-017-3652-1 |
| 26 | Liong MT . Beneficial microorganisms in medical and health applications[M]. Switzerland: Springer International Publishing, 2015. |
| [1] | Jia-he ZHANG,Jia-qi SHI,Zhang-jian CHEN,Guang JIA. Effects of nano titanium dioxide on gut microbiota based on human digestive tract microecology simulation system in vitro [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 468-476. |
| [2] | WANG Zi-jing,LI Zai-ling. Characteristics of gastric microbiota in children with Helicobacter pylori infection family history [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1115-1121. |
| [3] | Mei-xiang ZHANG,Wen-zhi SHI,Jian-xin LIU,Chun-jian WANG,Yan LI,Wei WANG,Bin JIANG. Clinical characteristics and prognosis of MLL-AF6 positive patients with acute myeloid leukemia [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 915-920. |
| [4] | YAN Yong-Qing, ZHANG Pei-Xun- , WANG Tian-Bing- , CHEN Jian-Hai, JIANG Bao-Guo. Multi-center retrospective analysis of clinical and related sociologic characteristics of postoperative distal radius fracture patients [J]. Journal of Peking University(Health Sciences), 2014, 46(2): 254-257. |
|
||