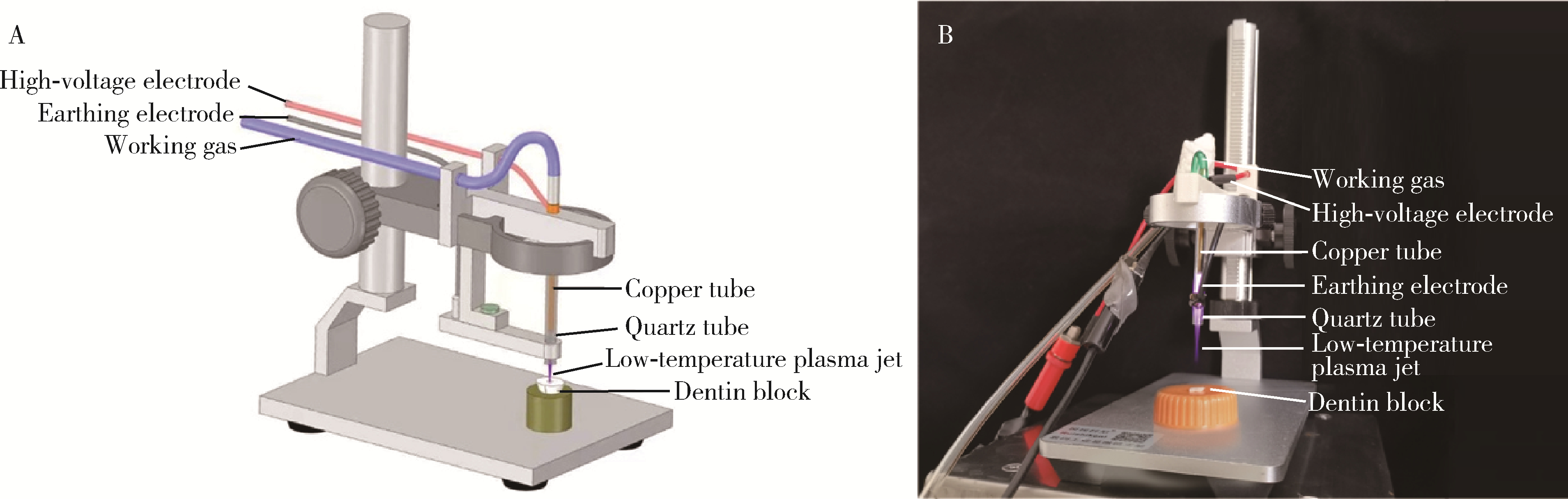
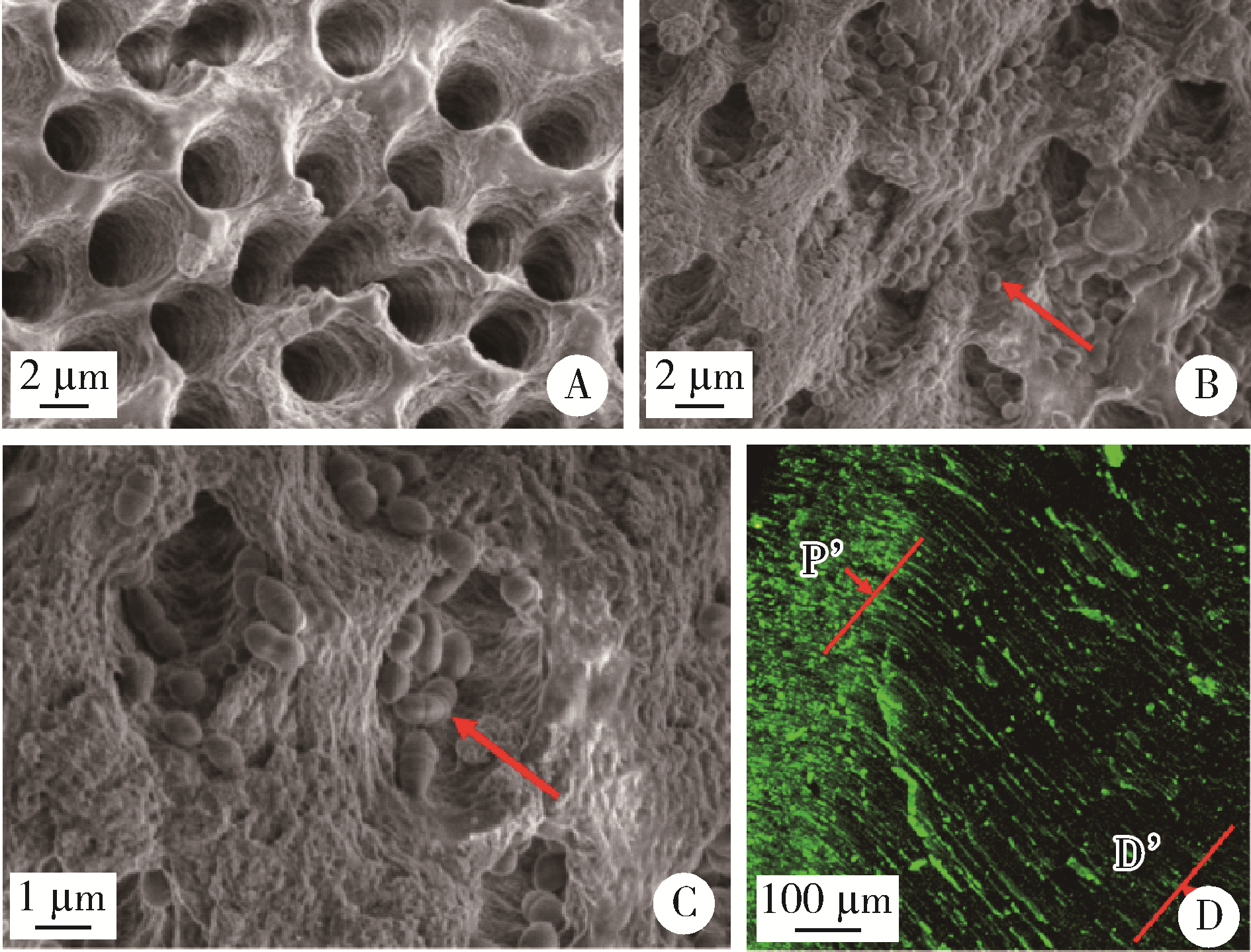
Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (1): 38-43. doi: 10.19723/j.issn.1671-167X.2023.01.006
Previous Articles Next Articles
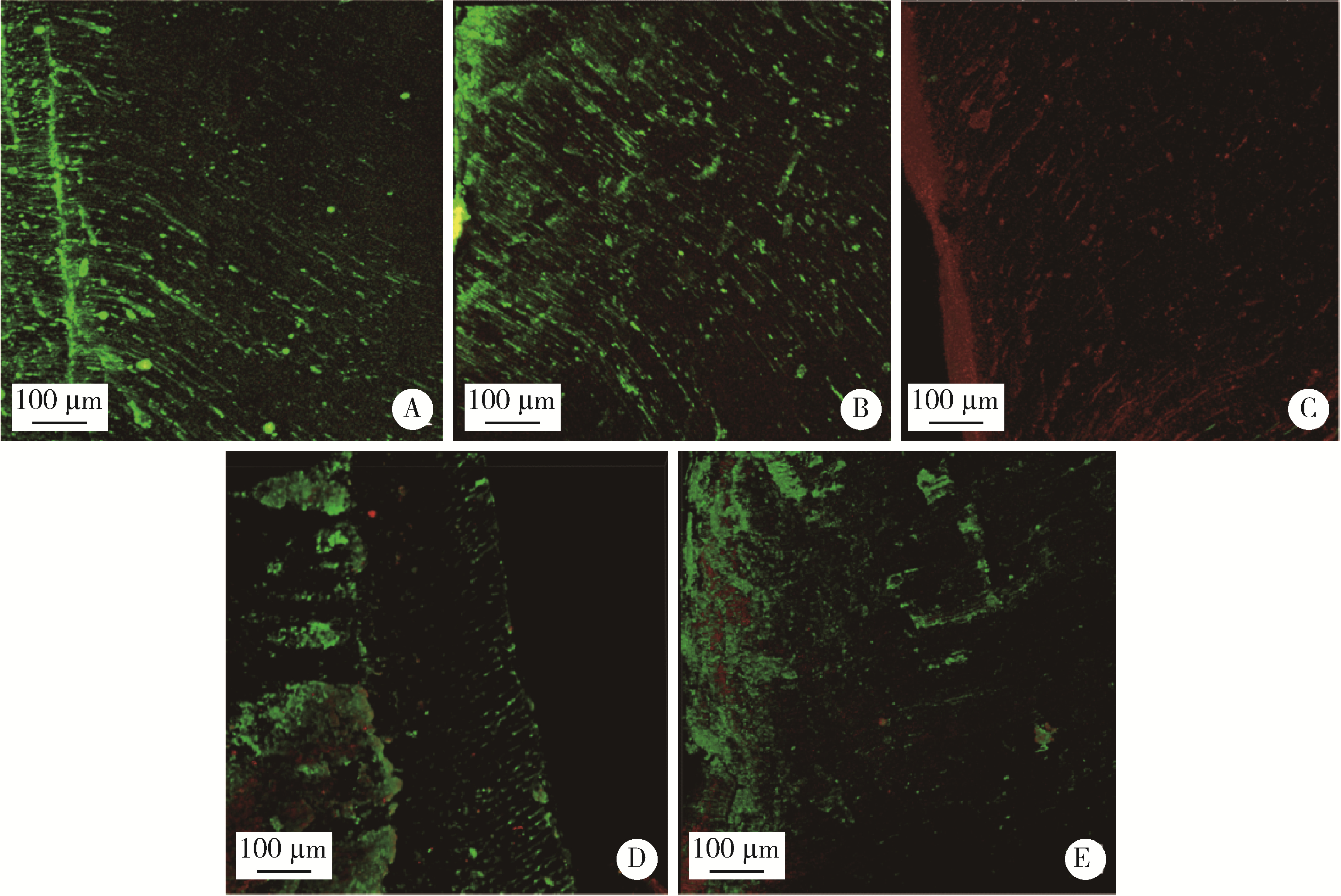
Antibacterial effect of low-temperature plasma on Enterococcus faecalis in dentinal tubules in vitro
Ruo-qing ZHONG1,Meng-qian ZHU1,Ying-long LI2,*( ),Jie PAN1,*(
),Jie PAN1,*( )
)
- 1. Department of General Dentistry, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. Department of Stomatology, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China
CLC Number:
- R781.3
| 1 | Friedman S , Mor C . The success of endodontic therapy: Healing and functionality[J]. J Calif Dent Assoc, 2004, 32 (6): 493- 503. |
| 2 |
Baras BH , Melo MAS , Sun J , et al. Novel endodontic sealer with dual strategies of dimethylaminohexadecyl methacrylate and nano-particles of silver to inhibit root canal biofilms[J]. Dent Mater, 2019, 35 (8): 1117- 1129.
doi: 10.1016/j.dental.2019.05.014 |
| 3 |
Stuart CH , Schwartz SA , Beeson TJ , et al. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment[J]. J Endod, 2006, 32 (2): 93- 98.
doi: 10.1016/j.joen.2005.10.049 |
| 4 |
Sundqvist G , Figdor D , Persson S , et al. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment[J]. Oral Surg Oral Med Oral Pathol Oral Radiol Endod, 1998, 85 (1): 86- 93.
doi: 10.1016/S1079-2104(98)90404-8 |
| 5 |
Chen ZT , Chen GJ , Obenchain R , et al. Cold atmospheric plasma delivery for biomedical applications[J]. Mater Today, 2022, 54, 153- 188.
doi: 10.1016/j.mattod.2022.03.001 |
| 6 |
Moreau M , Orange N , Feuilloley MG . Non-thermal plasma technologies: New tools for bio-decontamination[J]. Biotechnol Adv, 2008, 26 (6): 610- 617.
doi: 10.1016/j.biotechadv.2008.08.001 |
| 7 |
Chen K , Xu DG , Li JN , et al. Application of terahertz time-domain spectroscopy in atmospheric pressure plasma jet diagnosis[J]. Results Phys, 2020, 16, 102928.
doi: 10.1016/j.rinp.2020.102928 |
| 8 |
Tornin J , Labay C , Tampieri F , et al. Evaluation of the effects of cold atmospheric plasma and plasma-treated liquids in cancer cell cultures[J]. Nat Protoc, 2021, 16 (6): 2826- 2850.
doi: 10.1038/s41596-021-00521-5 |
| 9 |
Kumar-Dubey S , Dabholkar N , Narayan-Pal U , et al. Emerging innovations in cold plasma therapy against cancer: A paradigm shift[J]. Drug Discov Today, 2022, 27 (9): 2425- 2439.
doi: 10.1016/j.drudis.2022.05.014 |
| 10 |
Zhang H , Xu SD , Zhang JS , et al. Plasma-activated thermosensitive biogel as an exogenous ROS carrier for post-surgical treatment of cancer[J]. Biomaterials, 2021, 276, 121057.
doi: 10.1016/j.biomaterials.2021.121057 |
| 11 | Kim GC , Kim GJ , Park SR , et al. Air plasma coupled with antibody-conjugated nanoparticles: A new weapon against cancer[J]. J Phys D Appl Phys, 2008, 42 (3): 032005. |
| 12 |
Lu XP , Ye T , Cao YG , et al. The roles of the various plasma agents in the inactivation of bacteria[J]. J Appl Phys, 2008, 104 (5): 053309.
doi: 10.1063/1.2977674 |
| 13 |
Simon HU , Haj-Yehia A , Levi-Schaffer F . Role of reactive oxygen species (ROS) in apoptosis induction[J]. Apoptosis, 2000, 5 (5): 415- 418.
doi: 10.1023/A:1009616228304 |
| 14 |
Laroussi M . Low temperature plasma-based sterilization: Overview and state-of-the-art[J]. Plasma Process Polym, 2005, 2 (5): 391- 400.
doi: 10.1002/ppap.200400078 |
| 15 |
Pan J , Sun K , Liang YD , et al. Cold plasma therapy of a tooth root canal infected with Enterococcus faecalis biofilms in vitro[J]. J Endod, 2013, 39 (1): 105- 110.
doi: 10.1016/j.joen.2012.08.017 |
| 16 |
Li YL , Sun K , Ye GP , et al. Evaluation of cold plasma treatment and safety in disinfecting 3-week root canal Enterococcus faecalis biofilm in vitro[J]. J Endod, 2015, 41 (8): 1325- 1330.
doi: 10.1016/j.joen.2014.10.020 |
| 17 |
Ma JZ , Wang ZJ , Shen Y , et al. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy[J]. J Endod, 2011, 37 (10): 1380- 1385.
doi: 10.1016/j.joen.2011.06.018 |
| 18 | Du TF , Wang ZJ , Shen Y , et al. Effect of long-term exposure to endodontic disinfecting solutions on young and old Enterococcus faecalis biofilms in dentin canals[J]. J Endod, 2014, 40 (4): 509- 514. |
| 19 | 高岩, 李铁军. 口腔组织学与病理学[M]. 2版 北京: 北京大学医学出版社, 2013: 74. |
| 20 | 王雅丽, 罗丹, 申婷, 等. 不同冲洗方法对粪肠球菌感染根管的抗菌性研究[J]. 中华口腔医学研究杂志(电子版), 2019, 13 (4): 204- 211. |
| 21 | Li YL , Pan J , Wu D , et al. Regulation of Enterococcus faecalis biofilm formation and quorum sensing related virulence factors with ultra-low dose reactive species produced by plasma activated water[J]. Plasma Chem Plasma P, 2019, 39, 35- 49. |
| 22 | Liang YD , Li YL , Sun K , et al. Plasma thorns: Atmospheric pressure non-thermal plasma source for dentistry applications[J]. Plasma Process Polym, 2015, 12 (10): 1069- 1075. |
| 23 | Asnaashari M , Ashraf H , Rahmati A , et al. A comparison between effect of photodynamic therapy by LED and calcium hydro-xide therapy for root canal disinfection against Enterococcus faecalis: A randomized controlled trial[J]. Photodiagnosis Photodyn Ther, 2017, 17, 226- 232. |
| 24 | Afkhami F , Rostami G , Batebi S , et al. Residual antibacterial effects of a mixture of silver nanoparticles/calcium hydroxide and other root canal medicaments against Enterococcus faecalis[J]. J Dent Sci, 2022, 17 (3): 1260- 1265. |
| 25 | Gomes BP , Souza SF , Ferraz CC , et al. Effectiveness of 2% chlorhexidine gel and calcium hydroxide against Enterococcus faecalis in bovine root dentine in vitro[J]. Int Endod J, 2003, 36 (4): 267- 275. |
| 26 | Kapralos V , Sunde PT , Camilleri J , et al. Effect of chlorhexidine digluconate on antimicrobial activity, cell viability and physicochemical properties of three endodontic sealers[J]. Dent Mater, 2022, 38 (6): 1044- 1059. |
| [1] | Xiaoyi ZHAO,Chang LIU,Kun QIAN,Jie PAN. Efficacy and radiology evaluation of pulpotomy in mature permanent teeth [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 138-143. |
| [2] | Peiyao HE,Xudong BAO. Sealing effect of GuttaFlow2 in curved root canals [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 99-105. |
| [3] | Yu-yang YE,Lin YUE,Xiao-ying ZOU,Xiao-yan WANG. Characteristics and microRNA expression profile of exosomes derived from odontogenic dental pulp stem cells [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 689-696. |
| [4] | Jia-xue YE,Yu-hong LIANG. A prevalence survey of cone-beam computed tomography use among endodontic practitioners [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 114-119. |
| [5] | QIAN Kun,PAN Jie,ZHU Wen-hao,ZHAO Xiao-yi,LIU Chang,YONG Wei. Evaluation of bioceramic putty repairmen iRoot and mineral trioxide aggregate in mature permanent teeth pulpotomy [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 113-118. |
| [6] | WANG Zheng,DING Qian,GAO Yuan,MA Quan-quan,ZHANG Lei,GE Xi-yuan,SUN Yu-chun,XIE Qiu-fei. Effect of porous zirconia ceramics on proliferation and differentiation of osteoblasts [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 31-39. |
| [7] | MENG Yuan,ZHANG Li-qi,ZHAO Ya-ning,LIU Deng-gao,ZHANG Zu-yan,GAO Yan. Three-dimentional radiographic features of 67 maxillary radicular cysts [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 396-401. |
| [8] | Fei LI,Jing QIAO,Jin-yu DUAN,Yong ZHANG,Xiu-jing WANG. Effect of concentrated growth factors combined with guided tissue regeneration in treatment of classⅡ furcation involvements of mandibular molars [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 346-352. |
| [9] | Jing-yi LI,Sai-nan WANG,Yan-mei DONG. Anti-inflammatory and repaired effects of non-steroidal anti-inflammatory drugs on human dental pulp cells [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 24-29. |
| [10] | Ying-yi CHEN,Zi-qi HU,Tian-qian HUI,He LIU. Enhancer of zeste homolog 2 affects dental pulp inflammation by regulating macrophage chemotaxis [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 18-23. |
| [11] | Jia-sha WANG,Pei-yu WANG,Yu-hong LIANG. Effects of different methods on drying root canal by near-field microwave detection system [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1124-1129. |
| [12] | Qian-li ZHANG,Chong-yang YUAN,Li LIU,Shi-peng WEN,Xiao-yan WANG. Effects of electrospun collagen nanofibrous matrix on the biological behavior of human dental pulp cells [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 28-34. |
| [13] | Yue LEI,Ying-ting YANG,Yuan ZHAN. Evaluation of bioceramic putty repairment in primary molars pulpotomy [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 70-74. |
| [14] | LONG Yun-zi,LIU Si-yi, LI Wen, DONG Yan-mei. Physical and chemical properties of pulp capping materials based on bioactive glass [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 887-891. |
| [15] | LI Shuang, ZHANG Qing. Effect of smear layer on apical sealing ability of mineral trioxide aggregate (MTA) Plus through the sucrose penetration mode [J]. Journal of Peking University(Health Sciences), 2018, 50(3): 560-563. |
|
||