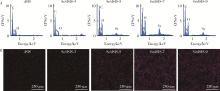
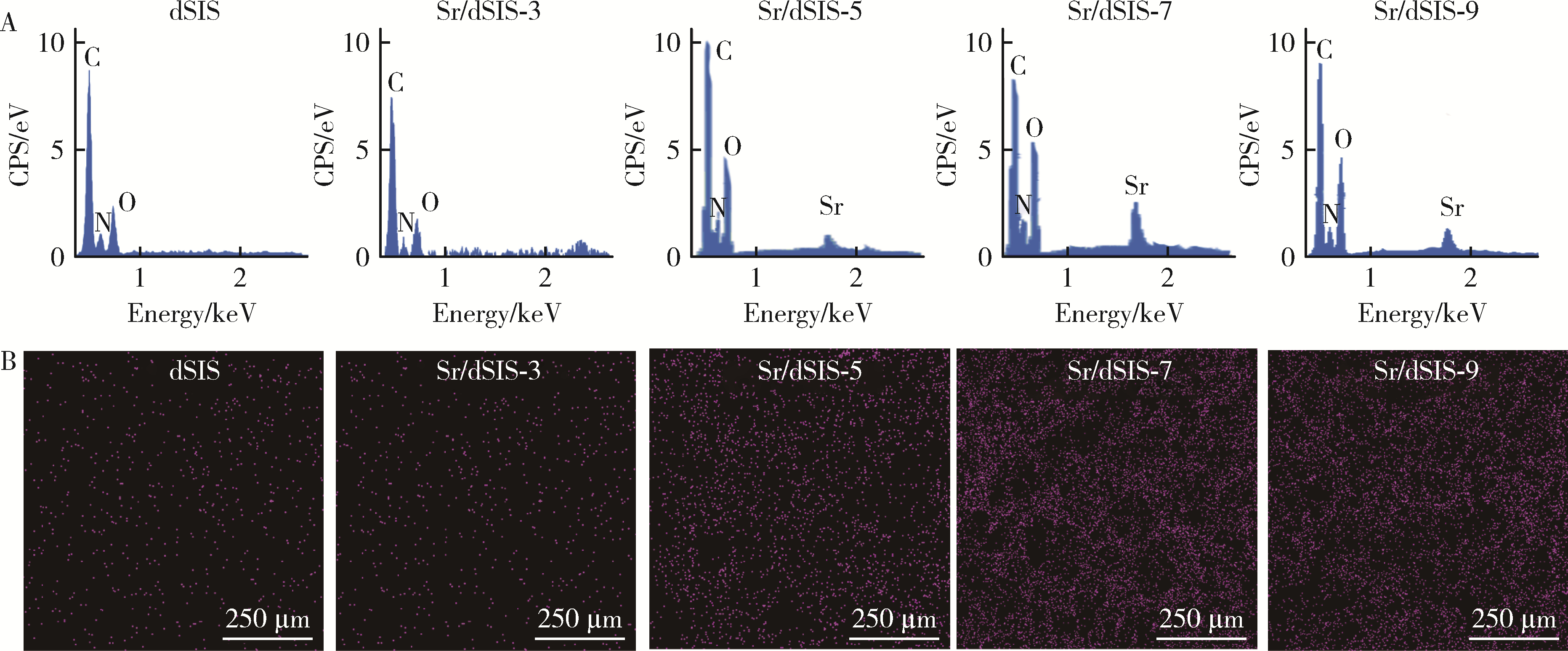
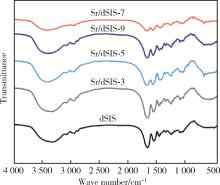
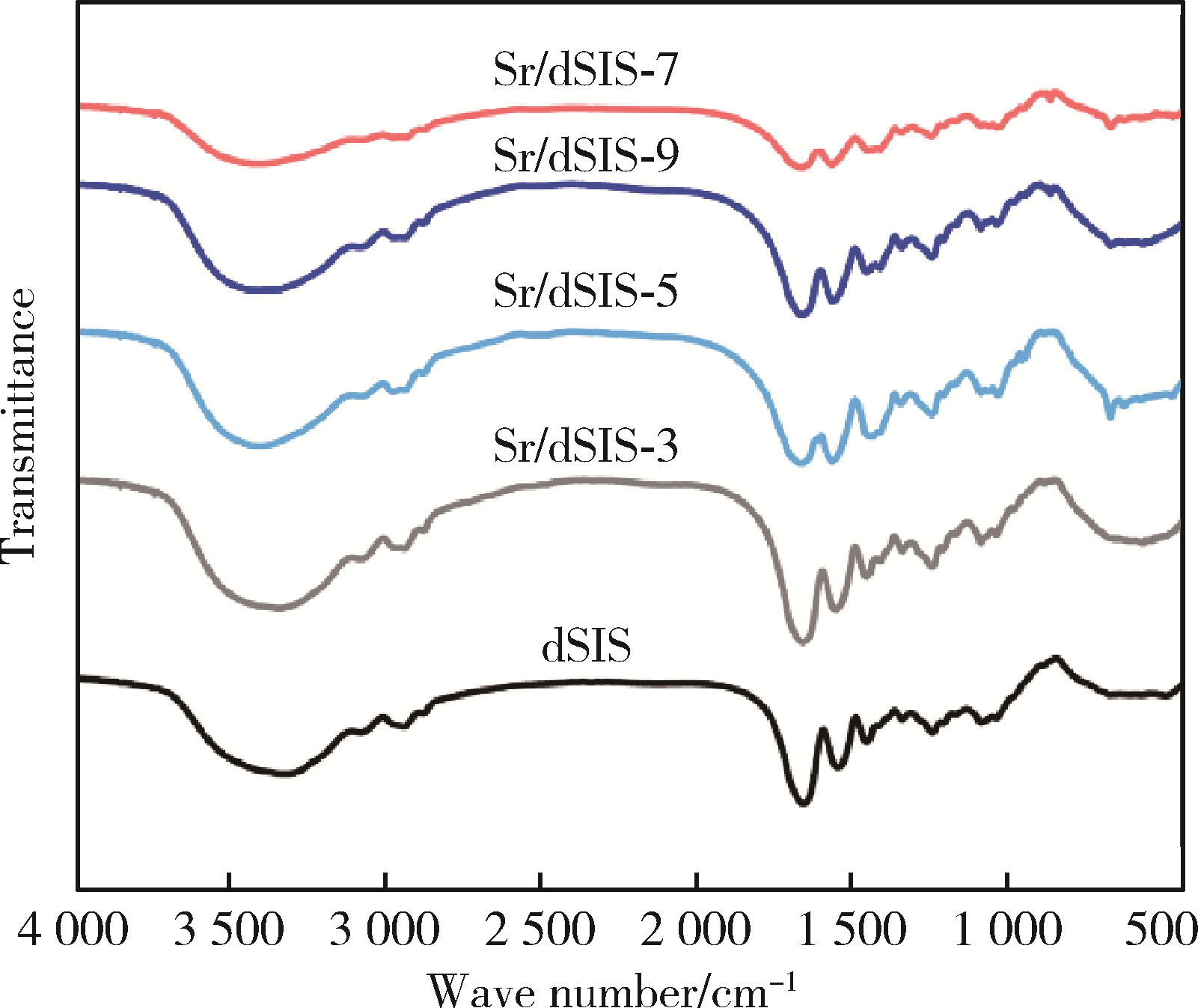
Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (1): 44-51. doi: 10.19723/j.issn.1671-167X.2023.01.007
Previous Articles Next Articles
Effect of pH on the chelation between strontium ions and decellularized small intestinal submucosal sponge scaffolds
Yu-ke LI1,Mei WANG2,Lin TANG1,Yu-hua LIU1,*( ),Xiao-ying CHEN1
),Xiao-ying CHEN1
- 1. Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. Department of Stomatology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing 100020, China
CLC Number:
- R318.08
| 1 |
Andrée B , Bär A , Haverich A , et al. Small intestinal submucosa segments as matrix for tissue engineering: review[J]. Tissue Eng Part B Rev, 2013, 19 (4): 279- 291.
doi: 10.1089/ten.teb.2012.0583 |
| 2 | Tian Q , Fan Y , Hao L , et al. A comprehensive review of calcium and ferrous ions chelating peptides: Preparation, structure and transport pathways[J]. Crit Rev Food Sci Nutr, 2021, 61 (11): 1- 13. |
| 3 |
O'Neill E , Awale G , Daneshmandi L , et al. The roles of ions on bone regeneration[J]. Drug Discov Today, 2018, 23 (4): 879- 890.
doi: 10.1016/j.drudis.2018.01.049 |
| 4 | Wu W , He L , Liang Y , et al. Preparation process optimization of pig bone collagen peptide-calcium chelate using response surface methodology and its structural characterization and stability analysis[J]. Food Chem, 2019, 284 (30): 80- 89. |
| 5 |
蔡冰娜, 陈忻, 潘剑宇, 等. 响应面法优化鳕鱼皮胶原蛋白肽螯合铁工艺[J]. 食品科学, 2012, 33 (2): 48- 52.
doi: 10.3969/j.issn.1671-1513.2012.02.010 |
| 6 |
Bi J , Wang X , Zhou Y , et al. Preparation and characterization for peptide-chelated calcium of deer bone[J]. Food Sci Technol Res, 2018, 24 (4): 717- 728.
doi: 10.3136/fstr.24.717 |
| 7 |
韩克光, 甄守艳, 范华, 等. 钙螯合羊骨胶原多肽的制备及表征分析[J]. 农业工程学报, 2015, 31 (21): 301- 307.
doi: 10.11975/j.issn.1002-6819.2015.21.040 |
| 8 | Zhang H , Zhao L , Shen Q , et al. Preparation of cattle bone collagen peptides-calcium chelate and its structural characterization and stability[J]. LWT-Food Sci Technol, 2021, 144 (12): 111264. |
| 9 | 陆剑锋, 孟昌伟, 李进, 等. 斑点叉尾鱼骨胶原多肽螯合钙的制备及其特征[J]. 水产学报, 2012, 36 (2): 314- 320. |
| 10 |
Crapo PM , Gilbert TW , Badylak SF . An overview of tissue and whole organ decellularization processes[J]. Biomaterials, 2011, 32 (12): 3233- 3243.
doi: 10.1016/j.biomaterials.2011.01.057 |
| 11 |
Li B , Wang M , Liu Y , et al. Independent effects of structural optimization and resveratrol functionalization on extracellular matrix scaffolds for bone regeneration[J]. Colloids Surf B Biointerfaces, 2022, 212, 112370.
doi: 10.1016/j.colsurfb.2022.112370 |
| 12 |
Reing JE , Brown BN , Daly KA , et al. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds[J]. Biomaterials, 2010, 31 (33): 8626- 8633.
doi: 10.1016/j.biomaterials.2010.07.083 |
| 13 | Ji Y , Zhou J , Sun T , et al. Diverse preparation methods for small intestinal submucosa (SIS): Decellularization, components, and structure[J]. J Biomed Mater Res A, 2019, 107 (3): 689- 697. |
| 14 |
Cowles EA , Brailey LL , Gronowicz GA . Integrin-mediated signaling regulates AP-1 transcription factors and proliferation in osteoblasts[J]. J Biomed Mater Res, 2000, 52 (4): 725- 737.
doi: 10.1002/1097-4636(20001215)52:4<725::AID-JBM18>3.0.CO;2-O |
| 15 |
Yi S , Ding F , Gong L , et al. Extracellular matrix scaffolds for tissue engineering and regenerative medicine[J]. Curr Stem Cell Res Ther, 2017, 12 (3): 233- 246.
doi: 10.2174/1574888X11666160905092513 |
| 16 | Liao J , Xu B , Zhang R , et al. Applications of decellularized materials in tissue engineering: Advantages, drawbacks and current improvements, and future perspectives[J]. J Mater Chem B, 2020, 8 (44): 10023- 10049. |
| 17 | Gorschewsky O , Puetz A , Riechert K , et al. Quantitative analysis of biochemical characteristics of bone-patellar tendon-bone allografts[J]. Biomed Mater Eng, 2005, 15 (6): 403- 411. |
| 18 | Bharadwaz A , Jayasuriya AC . Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration[J]. Mater Sci Eng C Mater Biol Appl, 2020, 110, 110698. |
| 19 | Chandika P , Ko SC , Oh GW , et al. Fish collagen/alginate/chitooligosaccharides integrated scaffold for skin tissue regeneration application[J]. Int J Biol Macromol, 2015, 81 (8): 504- 513. |
| 20 | Castilla Bolaños MA , Buttigieg J , Briceño Triana JC . Development and characterization of a novel porous small intestine submucosa-hydroxyapatite scaffold for bone regeneration[J]. Mater Sci Eng C Mater Biol Appl, 2017, 72, 519- 525. |
| 21 | Liu J , Zeng H , Xiao P , et al. Sustained release of magnesium ions mediated by a dynamic mechanical hydrogel to enhance BMSC proliferation and differentiation[J]. ACS Omega, 2020, 5 (38): 24477- 24486. |
| [1] | Xiaoying CHEN,Yi ZHANG,Yuke LI,Lin TANG,Yuhua LIU. Effects of different polymers on biomimetic mineralization of small intestine submucosal scaffolds [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 17-24. |
| [2] | Jiayun DONG,Xuefen LI,Ruifang LU,Wenjie HU,Huanxin MENG. Histopathological characteristics of peri-implant soft tissue in reconstructed jaws with vascularized bone flaps [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 25-31. |
| [3] | Han ZHAO,Yan WEI,Xuehui ZHANG,Xiaoping YANG,Qing CAI,Chengyun NING,Mingming XU,Wenwen LIU,Ying HUANG,Ying HE,Yaru GUO,Shengjie JIANG,Yunyang BAI,Yujia WU,Yusi GUO,Xiaona ZHENG,Wenjing LI,Xuliang DENG. Bionic design, preparation and clinical translation of oral hard tissue restorative materials [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 4-8. |
| [4] | Ying ZHOU,Ning ZHAO,Hongyuan HUANG,Qingxiang LI,Chuanbin GUO,Yuxing GUO. Application of double-layer soft tissue suture closure technique in the surgical treatment of patients with mandible medication-related osteonecrosis of the jaw of early and medium stages [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 51-56. |
| [5] | Yuan PAN,Hang GU,Han XIAO,Lijun ZHAO,Yiman TANG,Wenshu GE. Ubiquitin-specific protease 42 regulates osteogenic differentiation of human adipose-derived stem cells [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 9-16. |
| [6] | Xiaoqiang LIU,Yin ZHOU. Risk factors of perioperative hypertension in dental implant surgeries with bone augmentation [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 93-98. |
| [7] | Deng-hui DUAN,Hom-Lay WANG,En-bo WANG. Role of collagen membrane in modified guided bone regeneration surgery using buccal punch flap approach: A retrospective and radiographical cohort study [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1097-1104. |
| [8] | Da-wei WANG,Hua-dong WANG,Li LI,Xin YIN,Wei HUANG,Ji-dong GUO,Ya-feng YANG,Yi-hao LIU,Yang ZHENG. Efficacy analysis of autologous facet joint bone block in lumbar interbody fusion of osteoporosis patients [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 899-909. |
| [9] | Yi DENG,Yi ZHANG,Bo-wen LI,Mei WANG,Lin TANG,Yu-hua LIU. Effects of different crosslinking treatments on the properties of decellularized small intestinal submucosa porous scaffolds [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 557-564. |
| [10] | HE Wei,YANG Si-wen,CHEN Juan,ZHU Xiao-jun,CHEN Zhi-zhong,MA Wen-jun. Effects of 275 nm and 310 nm ultraviolet irradiation on bone metabolism in ovariectomized osteoporotic rats [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 236-243. |
| [11] | SHUAI Ting,LIU Juan,GUO Yan-yan,JIN Chan-yuan. Knockdown of long non-coding RNA MIR4697 host gene inhibits adipogenic differentiation in bone marrow mesenchymal stem cells [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 320-326. |
| [12] | LAN Lin,HE Yang,AN Jin-gang,ZHANG Yi. Relationship between prognosis and different surgical treatments of zygomatic defects: A retrospective study [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 356-362. |
| [13] | XUE Jiang,ZHANG Jian-yun,SHI Rui-rui,XIE Xiao-yan,BAI Jia-ying,LI Tie-jun. Clinicopathological analysis of 105 patients with fibrous dysplasia of cranio-maxillofacial region [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 54-61. |
| [14] | ZHONG Hua,XU Li-ling,BAI Ming-xin,SU Yin. Effect of chemokines CXCL9 and CXCL10 on bone erosion in patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1026-1031. |
| [15] | Yu YIN,Yu MEI,Ze-gang WANG,Shou-yi SONG,Peng-fei LIU,Peng-feng HE,Wen-jie WU,Xing XIE. Lengths of the fixed loop and the adjustable loop in the coarse bone tunnel were compared to influence the widening of the femoral bone tunnel and the function of the knee joint [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 883-890. |
|
||