Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (2): 315-323. doi: 10.19723/j.issn.1671-167X.2023.02.016
Previous Articles Next Articles
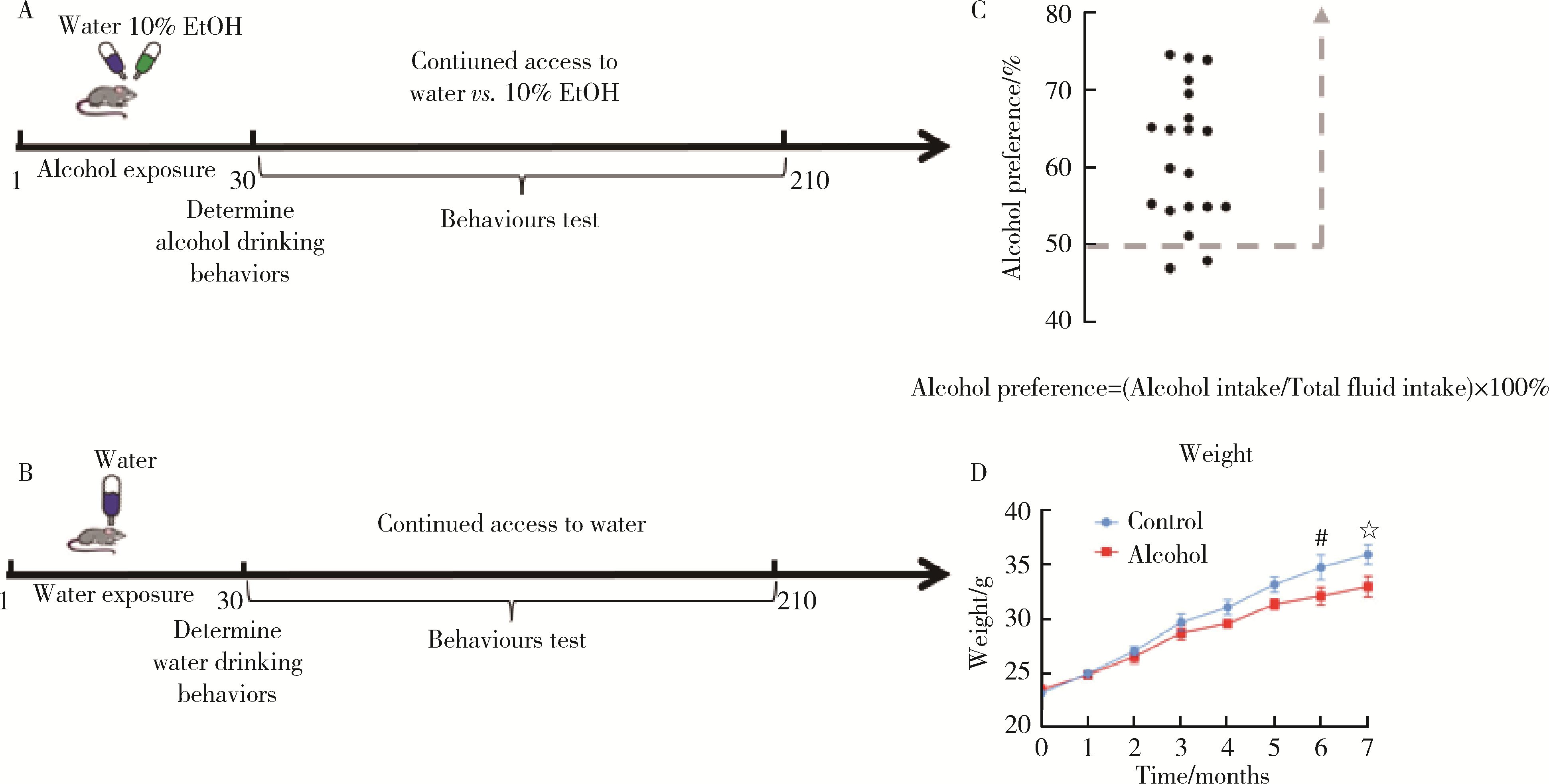
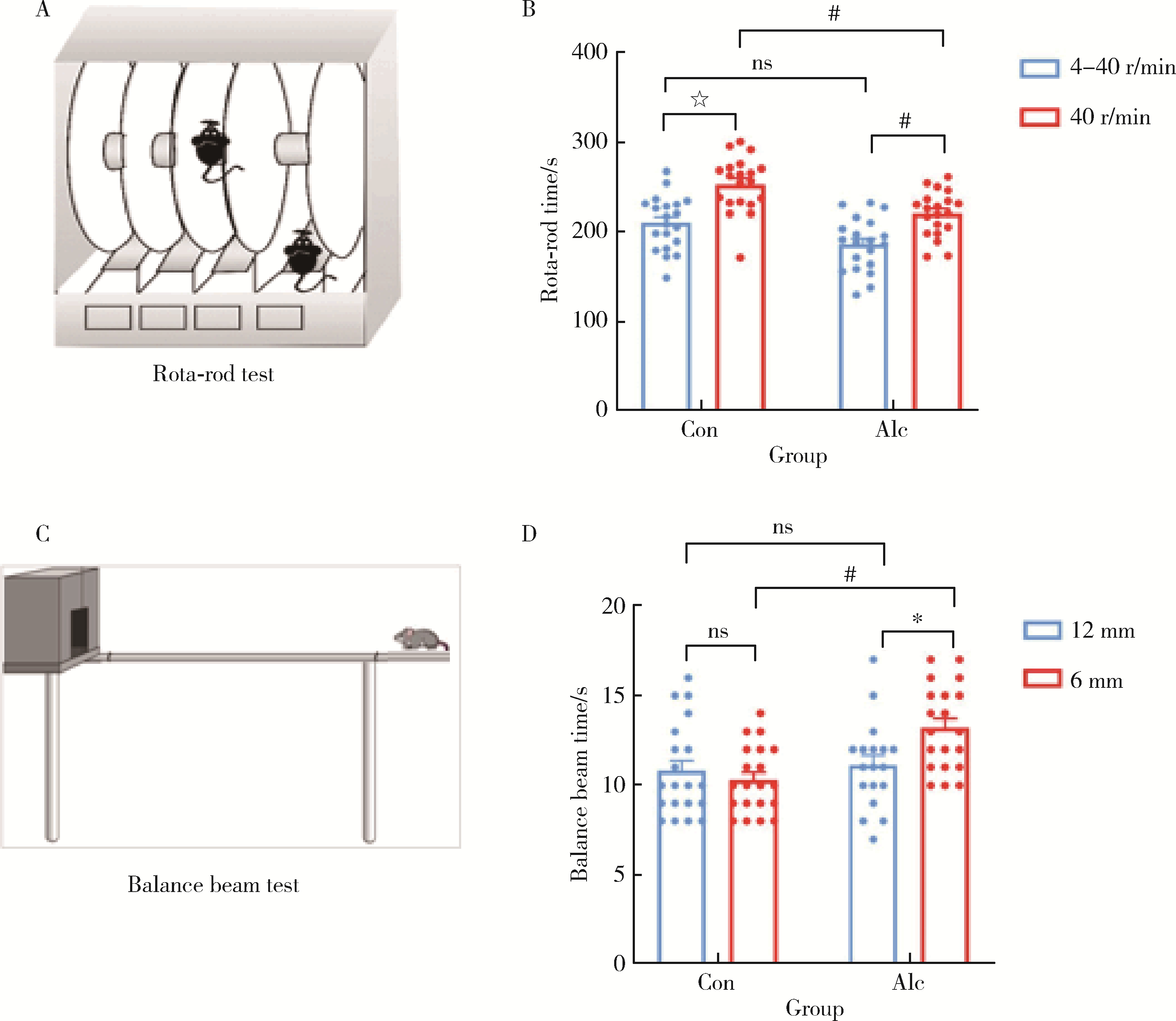
Establishment and behavioral evaluation of a mouse model of long-term free-choice alcohol drinking
Ting-ting YUAN1,Shen LI2,Yan WU2,Hai-tao WU1,2,*( )
)
- 1. Graduate Collaborative Training Base of Academy of Military Sciences, Hengyang Medical school, University of South China, Hengyang 421001, Hunan, China
2. Institute of Military Cognition and Brain Sciences, Academy of Military Medical Sciences, Academy of Military Sciences, Beijing 100850, China
CLC Number:
- R749.62
| 1 |
Wang SC , Chen YC , Chen SJ , et al. Alcohol addiction, gut microbiota, and alcoholism treatment: A review[J]. Int J Mol Sci, 2020, 21 (17): 6413.
doi: 10.3390/ijms21176413 |
| 2 |
Bree M . Combining research approaches to advance our understanding of drug addiction[J]. Current Psychiatry Reports, 2005, 7 (2): 125- 132.
doi: 10.1007/s11920-005-0009-4 |
| 3 |
Lemma A , Salelew E , Demilew D , et al. Alcohol use disorder and associated factors among University of Gondar undergraduate students: A cross-sectional study[J]. J Subst Abuse Treat, 2021, 129, 108373.
doi: 10.1016/j.jsat.2021.108373 |
| 4 |
Dewari PS , Ajani F , Kushawah G , et al. Reversible loss of reproductive fitness in zebrafish on chronic alcohol exposure[J]. Alcohol, 2016, 50, 83- 89.
doi: 10.1016/j.alcohol.2015.11.006 |
| 5 |
Iyer N , Vaishnava S . Alcohol lowers your (intestinal) inhibitions[J]. Cell Host Microbe, 2016, 19 (2): 131- 133.
doi: 10.1016/j.chom.2016.01.014 |
| 6 |
Hwa LS , Chu A , Levinson SA , et al. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol[J]. Alcohol Clin Exp Res, 2011, 35 (11): 1938- 1947.
doi: 10.1111/j.1530-0277.2011.01545.x |
| 7 |
Marcus DJ , Henderson-Redmond AN , Gonek M , et al. Mice expressing a "hyper-sensitive" form of the CB1 cannabinoid receptor (CB1) show modestly enhanced alcohol preference and consumption[J]. PLoS One, 2017, 12 (4): e0174826.
doi: 10.1371/journal.pone.0174826 |
| 8 |
王亚南, 刘儒林, 程秀臻. 酒精中毒大鼠动物模型的研究[J]. 中国康复理论与实践, 2006, 12 (4): 319- 320.
doi: 10.3969/j.issn.1006-9771.2006.04.017 |
| 9 |
Okazaki S , Nagoya S , Tateda K , et al. Experimental rat model for alcohol-induced osteonecrosis of the femoral head[J]. Int J Exp Pathol, 2013, 94 (5): 312- 319.
doi: 10.1111/iep.12035 |
| 10 |
Wang J , Du H , Jiang L , et al. Oxidation of ethanol in the rat brain and effects associated with chronic ethanol exposure[J]. Proc Natl Acad Sci USA, 2013, 110 (35): 14444- 14449.
doi: 10.1073/pnas.1306011110 |
| 11 |
Xu GF , Wang XY , Ge GL , et al. Dynamic changes of capillarization and peri-sinusoid fibrosis in alcoholic liver diseases[J]. World J Gastroenterol, 2004, 10 (2): 238- 243.
doi: 10.3748/wjg.v10.i2.238 |
| 12 |
Scott H , Tjernstrom N , Roman . Effects of pair housing on voluntary alcohol intake in male and female Wistar rats[J]. Alcohol, 2020, 86, 121- 128.
doi: 10.1016/j.alcohol.2019.12.005 |
| 13 |
Juarez B , Morel C , Ku SM , et al. Author correction: Midbrain circuit regulation of individual alcohol drinking behaviors in mice[J]. Nat Commun, 2018, 9 (1): 653.
doi: 10.1038/s41467-018-02921-w |
| 14 |
Romao-Torres M , Feranández-Guasti A . Estradiol valerate elicits antidepressant-like effects in middle-aged female rats under chronic mild stress[J]. Behav Pharmacol, 2010, 21 (2): 104- 111.
doi: 10.1097/FBP.0b013e328337bdfc |
| 15 |
Song YN , Li HZ , Zhu JN , et al. Histamine improves rat rota-rod and balance beam performances through H(2) receptors in the cerebellar interpositus nucleus[J]. Neuroscience, 2006, 140 (1): 33- 43.
doi: 10.1016/j.neuroscience.2006.01.045 |
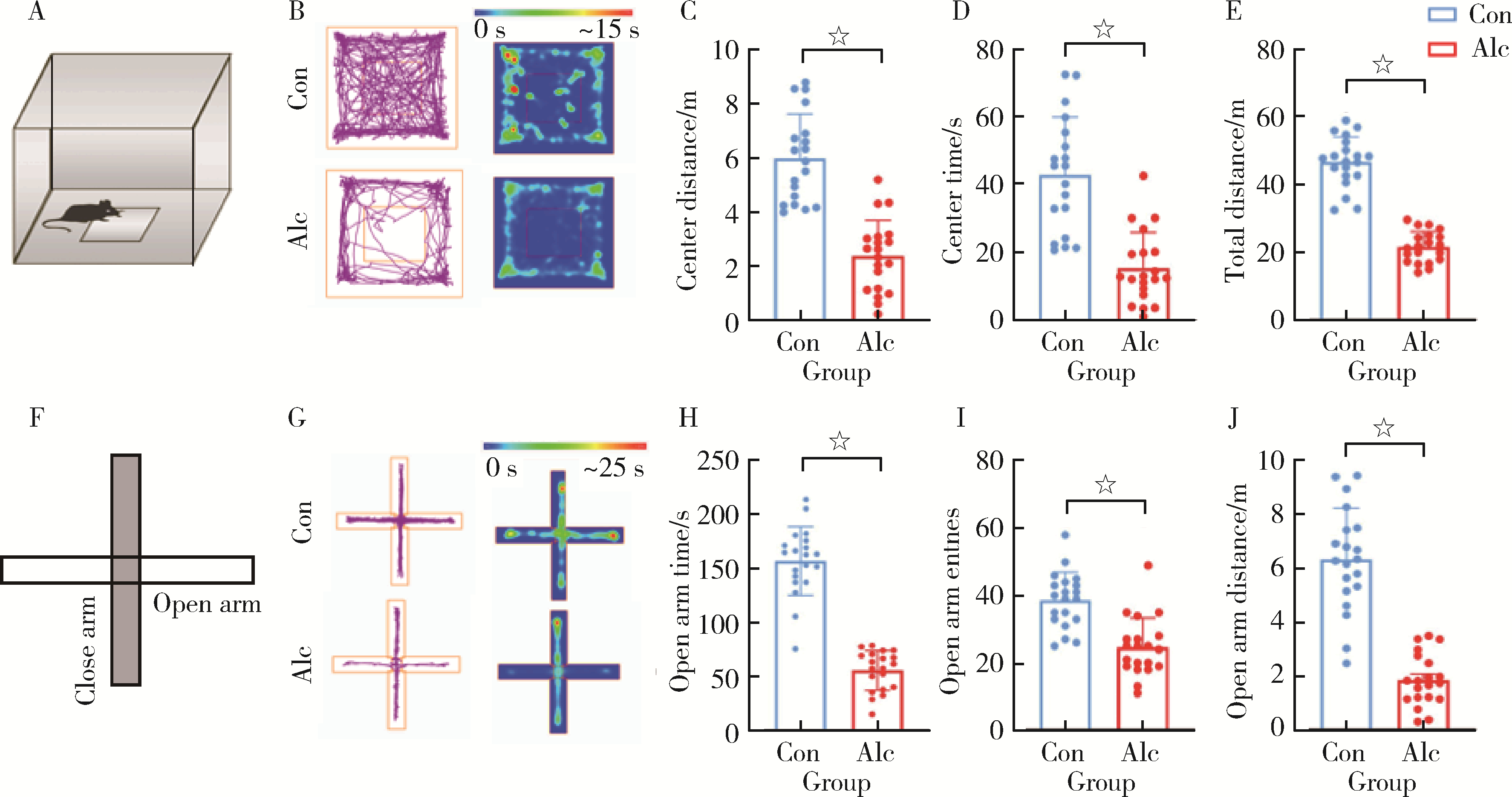
| 16 | Kraeuter AK , Guest PC , Sarnyai Z . The open field test for measuring locomotor activity and anxiety-like behavior[J]. Methods Mol Biol, 2019, 1916, 99- 103. |
| 17 | Kraeuter AK , Guest PC , Sarnyai Z . The elevated plus maze test for measuring anxiety-like behavior in rodents[J]. Methods Mol Biol, 2019, 1916, 69- 74. |
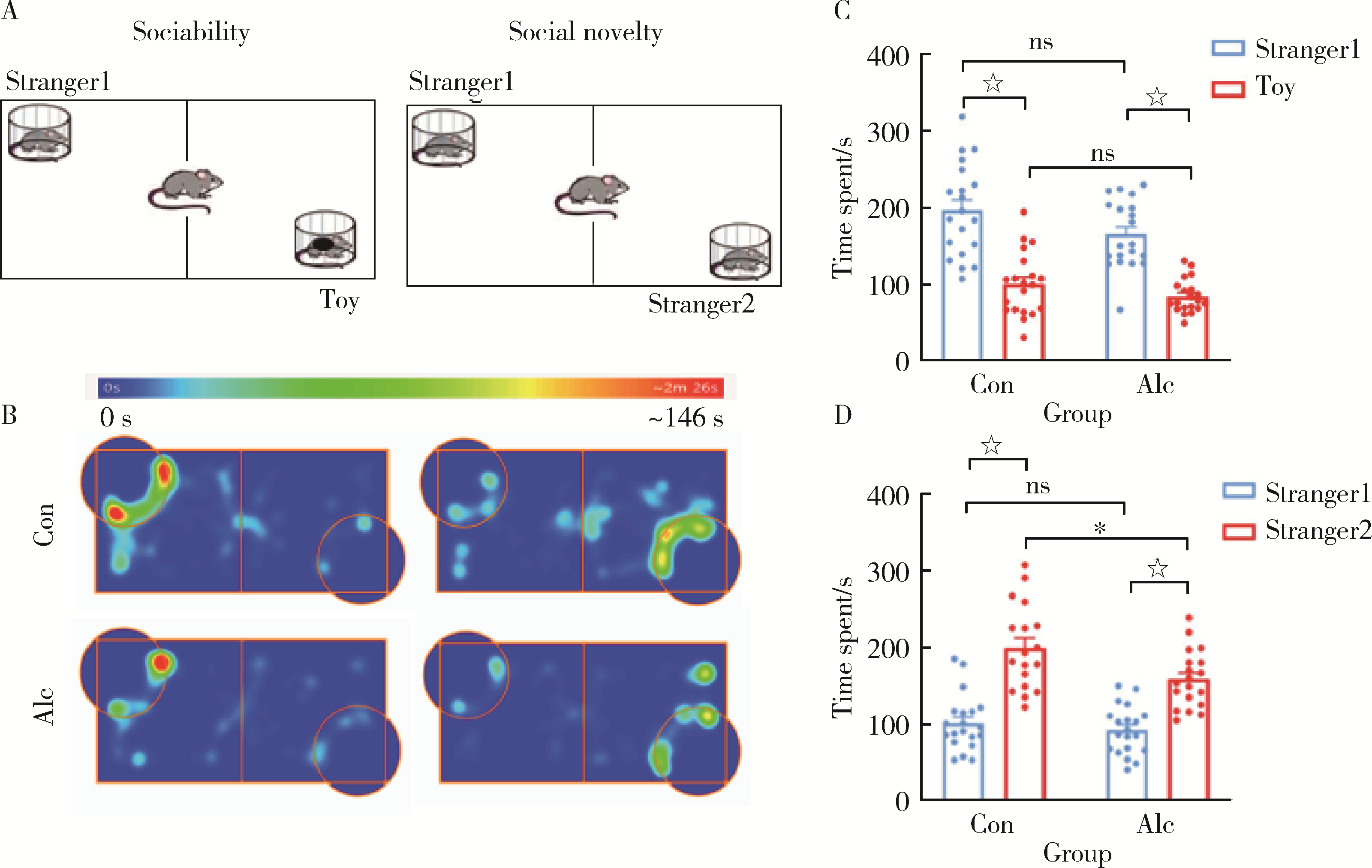
| 18 | 吴海涛, 赵哲. 一种小鼠社交行为研究用实验箱: 中国, CN110301364B[P/OL]. 2019-08-02[2021-09-24]. https://pss-system.cponline.cnipa.gov.cn/documents/detail?prevPageTit=changgui. |
| 19 |
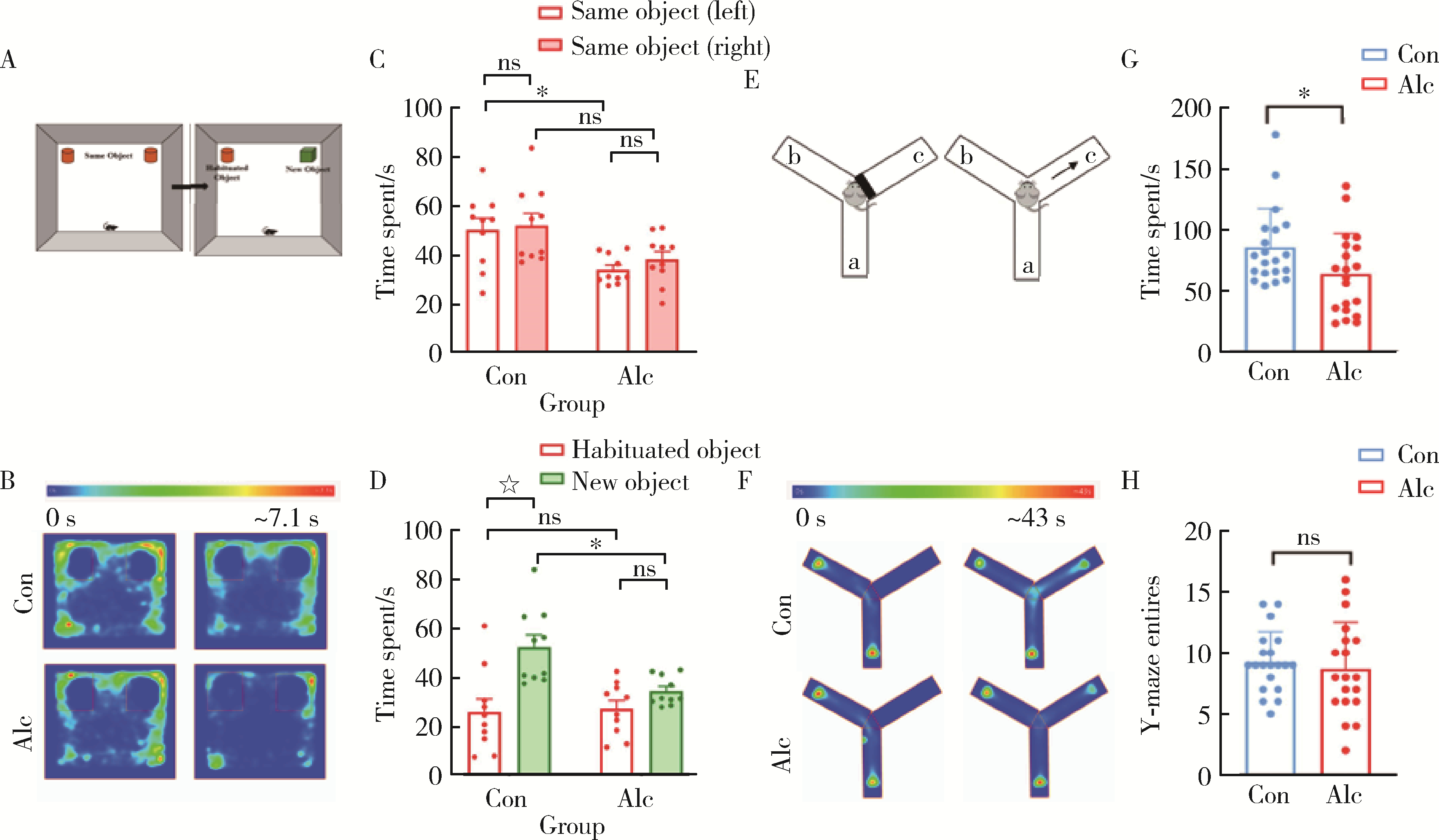
Bevins RA , Besheer J . Object recognition in rats and mice: A one-trial non-matching-to-sample learning task to study 'recognition memory'[J]. Nat Protoc, 2006, 1 (3): 1306- 1311.
doi: 10.1038/nprot.2006.205 |
| 20 |
Fu Y , Wang C , Wang J , et al. Long-term exposure to extremely low-frequency magnetic fields impairs spatial recognition memory in mice[J]. Clin Exp Pharmacol Physiol, 2008, 35 (7): 797- 800.
doi: 10.1111/j.1440-1681.2008.04922.x |
| 21 |
Martin S , Jones M , Simpson E , et al. Impaired spatial reference memory in aromatase-deficient (ArKO) mice[J]. Neuroreport, 2003, 14 (15): 1979- 1982.
doi: 10.1097/00001756-200310270-00020 |
| 22 |
Bejar R , Yasuda R , Krugers H , et al. Transgenic calmodulin-dependent protein kinase Ⅱ activation: Dose-dependent effects on synaptic plasticity, learning, and memory[J]. J Neurosci, 2002, 22 (13): 5719- 5726.
doi: 10.1523/JNEUROSCI.22-13-05719.2002 |
| 23 |
Qian L , Chen Y , Shen C , et al. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB[J]. Faseb J, 2017, 31 (4): 1494- 1507.
doi: 10.1096/fj.201601071R |
| 24 |
佟昕琪, 于鸣, 王泓, 等. 酒精对大鼠学习记忆能力的影响[J]. 吉林医药学院学报, 2021, 42 (3): 172- 175.
doi: 10.13845/j.cnki.issn1673-2995.2021.03.004 |
| 25 |
Inagawa G , Sato K , Kikuchi T , et al. Chronic ethanol consumption does not affect action of propofol on rat hippocampal acetylcholine release in vivo[J]. Br J Anaesth, 2004, 93 (5): 737- 739.
doi: 10.1093/bja/aeh263 |
| 26 |
Miller S , Yasuda M , Coats JK , et al. Disruption of dendritic translation of CaMKⅡalpha impairs stabilization of synaptic plasticity and memory consolidation[J]. Neuron, 2002, 36 (3): 507- 519.
doi: 10.1016/S0896-6273(02)00978-9 |
| 27 |
Wang J , Wang Z , Li B , et al. Lycopene attenuates western-diet-induced cognitive deficits via improving glycolipid metabolism dysfunction and inflammatory responses in gut-liver-brain axis[J]. Int J Obes (Lond), 2019, 43 (9): 1735- 1746.
doi: 10.1038/s41366-018-0277-9 |
| 28 |
Liu ZG , Sun Yali , Qiao QL , et al. Sesamol ameliorates high-fat and high-fructose induced cognitive defects via improving insulin signaling disruption in the central nervous system[J]. Food Funct, 2017, 8 (2): 710- 719.
doi: 10.1039/C6FO01562J |
| 29 |
Crupi R , Cambiaghi M , Spatz L , et al. Reduced adult neurogenesis and altered emotional behaviors in autoimmune-prone B-cell activating factor transgenic mice[J]. Biol psychiatry, 2010, 67 (6): 558- 566.
doi: 10.1016/j.biopsych.2009.12.008 |
| 30 |
Oury F , Khristine L , Denny CA , et al. Maternal and offspring pools of osteocalcin influence brain development and functions[J]. Cell, 2013, 155 (1): 228- 241.
doi: 10.1016/j.cell.2013.08.042 |
| 31 |
张富强, 唐甩恩, 周文华, 等. 程序诱导的大鼠酒精偏爱模型[J]. 中国药物滥用防治杂志, 2002, 14 (5): 14- 16.
doi: 10.3969/j.issn.1006-902X.2002.05.004 |
| 32 |
Goldstein DB , Pal N . Alcohol dependence produced in mice by inhalation of ethanol: Grading the withdrawal reaction[J]. Science, 1971, 172 (3980): 288- 290.
doi: 10.1126/science.172.3980.288 |
| 33 | Tsukamoto H , Reidelberger RD , French SW , et al. Long-term cannulation model for blood sampling and intragastric infusion in the rat[J]. Am J physiol, 1984, 247 (2/3): R595- 599. |
| 34 | Lieber CS . Microsomal ethanol-oxidizing system[J]. Enzyme, 1987, 37 (1/2): 45- 56. |
| 35 | 陈韶华, 厉有名, 虞朝辉, 等. 急性乙醇中毒与内源性一氧化氮水平关系的实验研究[J]. 中国自然医学杂志, 2003, 5 (1): 24- 26. |
| 36 | In HS , Kim DW , Park YM , et al. Experimental intraperitoneal injection of alcohol in rats: Peritoneal findings and histopathology[J]. Toxicol Rep, 2014, 28 (1): 31- 35. |
| 37 |
Cui SQ , Wang Q , Zheng Y , et al. Puerarin protects against damage to spatial learning and memory ability in mice with chronic alcohol poisoning[J]. Braz J Med Biol Res, 2015, 48 (6): 515- 522.
doi: 10.1590/1414-431x20144250 |
| 38 |
Umhau J C , Zhou W , Thada S , et al. Brain docosahexaenoic acid[DHA] incorporation and blood flow are increased in chronic alcoholics: A positron emission tomography study corrected for cerebral atrophy[J]. PLoS One, 2013, 8 (10): e75333.
doi: 10.1371/journal.pone.0075333 |
| 39 |
Palm S , Nylander I . Alcohol-induced changes in opioid peptide levels in adolescent rats are dependent on housing conditions[J]. Alcohol Clin Exp Res, 2014, 38 (12): 2978- 2987.
doi: 10.1111/acer.12586 |
| 40 |
Ozsoy S , Durak AC , Esel E . Hippocampal volumes and cognitive functions in adult alcoholic patients with adolescent-onset[J]. Alcohol, 2013, 47 (1): 9- 14.
doi: 10.1016/j.alcohol.2012.09.002 |
| [1] | Meng-qiao PAN,Jian LIU,Li XU,Xiao XU,Jian-xia HOU,Xiao-tong LI,Xiao-xia WANG. A long-term evaluation of periodontal phenotypes before and after the periodontal-orthodontic-orthognathic combined treatment of lower anterior teeth in patients with skeletal Angle class Ⅲ malocclusion [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 52-61. |
| [2] | MU Dong-liang,XUE Cheng,AN Bin,WANG Dong-xin. Epidural block associated with improved long-term survival after surgery for colorectal cancer: A retrospective cohort study with propensity score matching [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1152-1158. |
| [3] | DAI Xiang,ZUO Mei-ni,ZHANG Xiao-peng,HU Hao,XU Tao. Comparison of long-term outcomes in different managements of diverticular neck in percutaneous nephrolithotomy for diverticular calculi [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 704-709. |
| [4] | Ming-rui WANG,Qi WANG,Hao HU,Jin-hui LAI,Yong-xin HE,Jie XIONG,Xian-hui LIU,Shi-jun LIU,Ke-xin XU,Tao XU. Long-term analysis of safety and efficacy of standard percutaneous nephrolithotomy in patients with solitary kidneys [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 663-666. |
| [5] | Shan-shan BAI,Si-yi MO,Xiao-xiang XU,Yun LIU,Qiu-fei XIE,Ye CAO. Characteristics of orofacial operant test for orofacial pain sensitivity caused by occlusal interference in rats [J]. Journal of Peking University(Health Sciences), 2020, 52(1): 51-57. |
|
||