Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (5): 908-912. doi: 10.19723/j.issn.1671-167X.2024.05.024
Previous Articles Next Articles
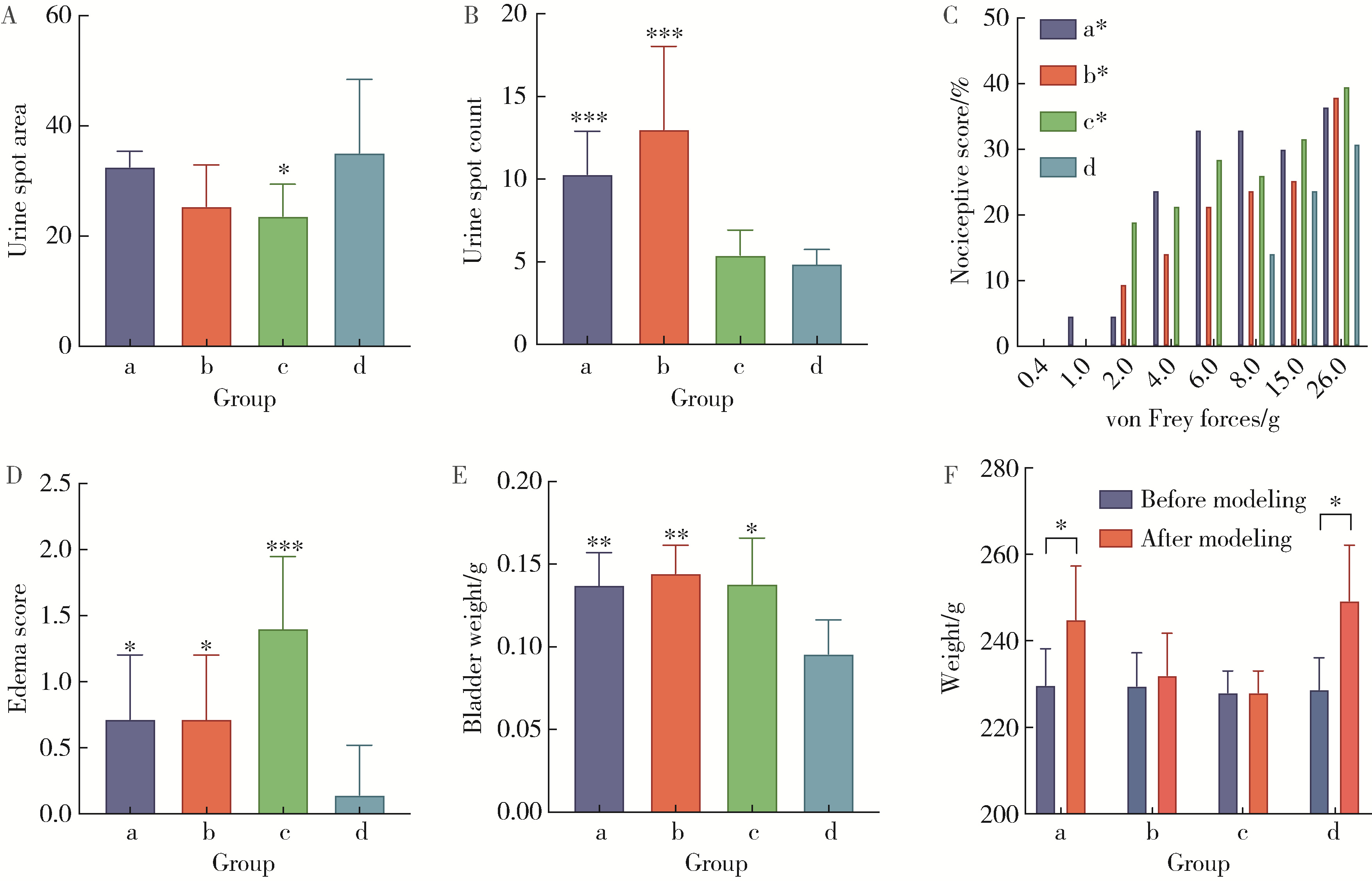
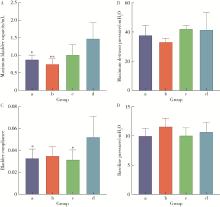
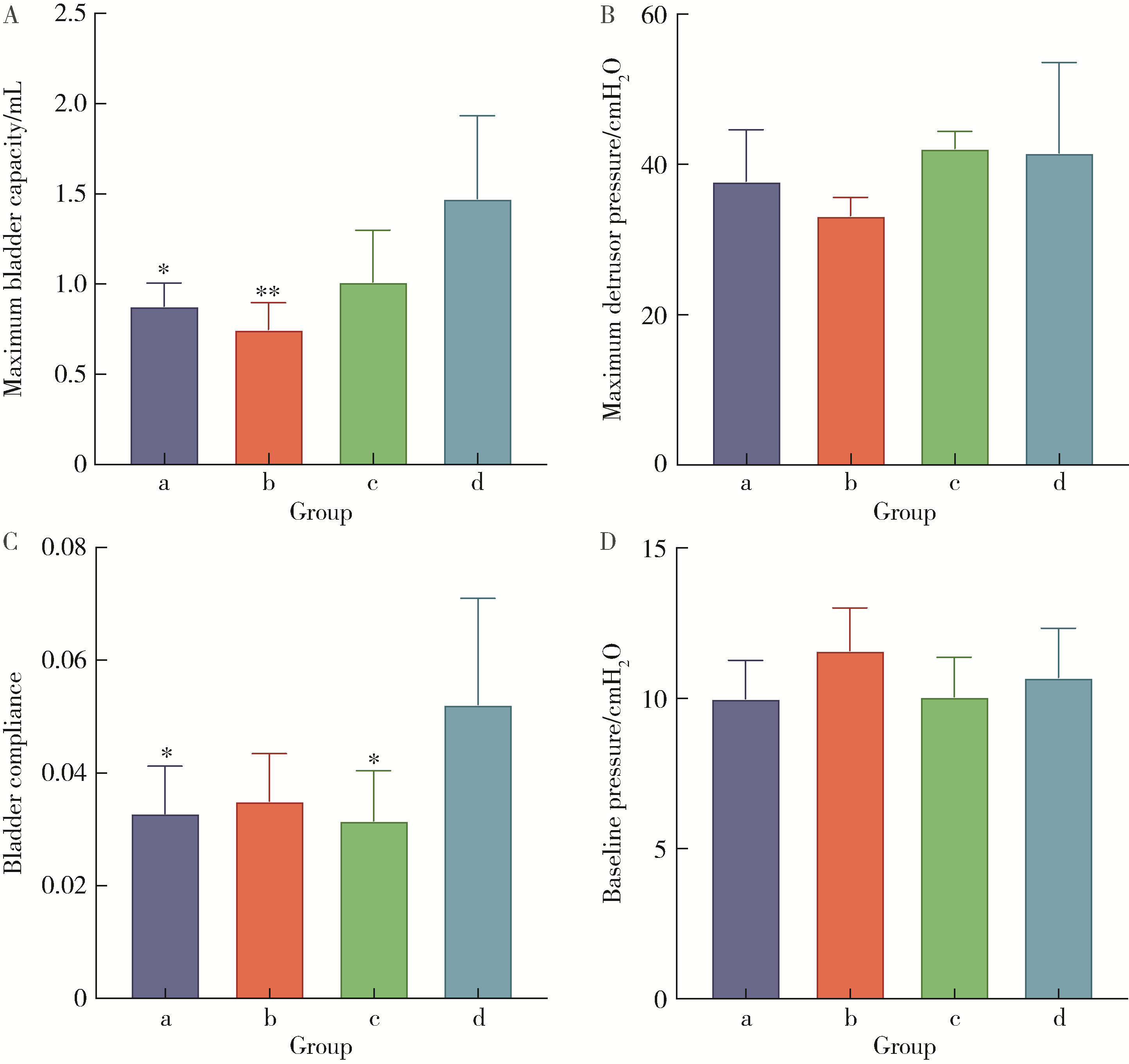
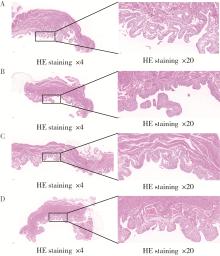
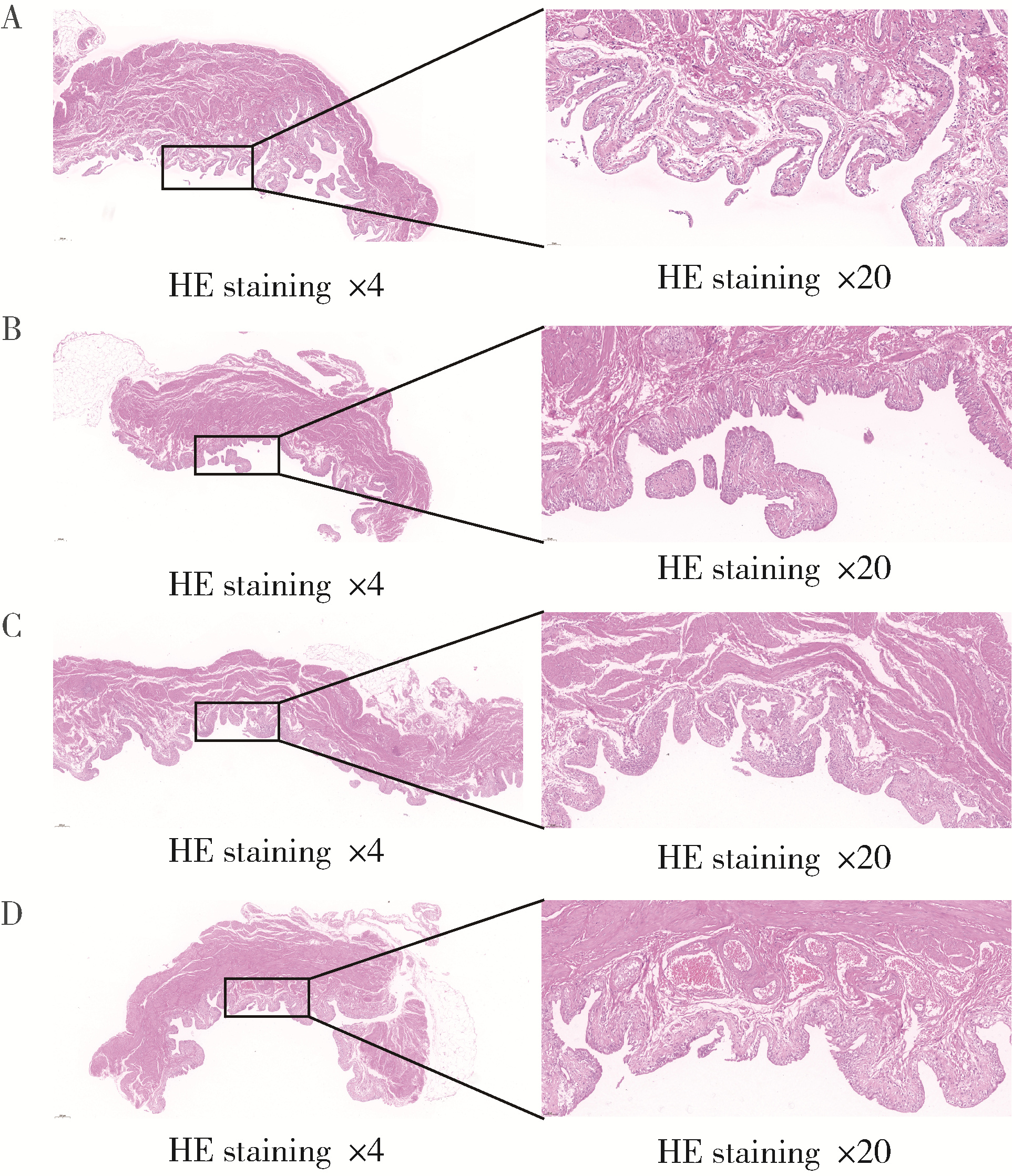
Optimization study of an animal model for interstitial cystitis/bladder pain syndrome based on the dose effect of cyclophosphamide
Hanwei KE, Qi WANG, Kexin XU*( )
)
- Department of Urology, Peking University Applied Liyhotripsy Institute, Peking University People's Hospital, Beijing 100044, China
CLC Number:
- R694.3
| 1 |
Driscoll A , Teichman JMH . How do patients with interstitial cystitis present[J]. J Urol, 2001, 166 (6): 2118- 2120.
doi: 10.1016/S0022-5347(05)65517-6 |
| 2 |
Hanno PM , Erickson D , Moldwin R , et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment[J]. J Urol, 2015, 193 (5): 1545- 1553.
doi: 10.1016/j.juro.2015.01.086 |
| 3 |
Parsons CL . The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis[J]. Urology, 2007, 69, S9- S16.
doi: 10.1016/j.urology.2006.03.084 |
| 4 |
van de Merwe JP . Interstitial cystitis and systemic autoimmune diseases[J]. Nat Clin Pract Urol, 2007, 4 (9): 484- 491.
doi: 10.1038/ncpuro0874 |
| 5 |
Cox PJ . Cyclophosphamide cystitis: Identification of acrolein as the causative agent[J]. Biochem Pharmacol, 1979, 28 (13): 2045- 2049.
doi: 10.1016/0006-2952(79)90222-3 |
| 6 |
Liu XY , Zhu MZ , Xie JP . Mutagenicity of acrolein and acrolein-induced DNA adducts[J]. Toxicol Mech Methods, 2010, 20 (1): 36- 44.
doi: 10.3109/15376510903530845 |
| 7 |
Vera PL , Iczkowski KA , Wang X , et al. Cyclophosphamide-induced cystitis increases bladder CXCR4 expression and CXCR4-macrophage migration inhibitory factor association[J]. PLoS One, 2008, 3 (12): e3898.
doi: 10.1371/journal.pone.0003898 |
| 8 |
Boucher M , Meen M , Codron JP , et al. Cyclophosphamide-induced cystitis in freely-moving conscious rats: Behavioral approach to a new model of visceral pain[J]. J Urol, 2000, 164 (1): 203- 208.
doi: 10.1016/S0022-5347(05)67495-2 |
| 9 | Augé C , Chene G , Dubourdeau M , et al. Relevance of the cyclophosphamide-induced cystitis model for pharmacological studies targeting inflammation and pain of the bladder[J]. Eur J Pharmacol, 2013, 707 (1/2/3): 32- 40. |
| 10 |
Augé C , Gamé X , Vergnolle N , et al. Characterization and validation of a chronic model of cyclophosphamide-induced interstitial cystitis/bladder pain syndrome in rats[J]. Front Pharmacol, 2020, 11, 1305.
doi: 10.3389/fphar.2020.01305 |
| 11 |
Gray KJ , Engelmann UH , Johnson EH , et al. Evaluation of misoprostol cytoprotection of the bladder with cyclophosphamide (Cytoxan) therapy[J]. J Urol, 1986, 136 (2): 497- 500.
doi: 10.1016/S0022-5347(17)44929-9 |
| 12 |
Hakimi Z , Houbiers J , Pedersini R , et al. The burden of bladder pain in five european countries: A cross-sectional study[J]. Urology, 2017, 99, 84- 91.
doi: 10.1016/j.urology.2016.08.038 |
| 13 |
Smaldone MC , Vodovotz Y , Tyagi V , et al. Multiplex analysis of urinary cytokine levels in rat model of cyclophosphamide-induced cystitis[J]. Urology, 2009, 73 (2): 421- 426.
doi: 10.1016/j.urology.2008.07.031 |
| 14 |
Arms L , Girard BM , Vizzard MA . Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis[J]. Am J Physiol Renal Physiol, 2010, 298 (3): F589- F600.
doi: 10.1152/ajprenal.00628.2009 |
| 15 |
May V , Vizzard MA . Bladder dysfunction and altered somatic sensitivity in PACAP-/-mice[J]. J Urol, 2010, 183 (2): 772- 779.
doi: 10.1016/j.juro.2009.09.077 |
| 16 |
Augé C , Basso L , Blanpied C , et al. Pain management in a model of interstitial cystitis/bladder pain syndrome by a vaccinal strategy[J]. Front Pain Res (Lausanne), 2021, 2, 642706.
doi: 10.3389/fpain.2021.642706 |
| 17 |
Koziol JA , Adams HP , Frutos A . Discrimination between the ulcerous and the nonulcerous forms of interstitial cystitis by noninvasive findings[J]. J Urol, 1996, 155 (1): 87- 90.
doi: 10.1016/S0022-5347(01)66551-0 |
| [1] | Peng XIN,Hao ZHANG,Zhen-ming JIANG. Treatment of intravesical instillation with fulguration-hydrodistention on female interstitial cystitis [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 865-870. |
| [2] | Ling-wei MENG,Xue LI,Sheng-han GAO,Yue LI,Rui-tao CAO,Yi ZHANG,Shao-xia PAN. Comparison of three methods for establishing rat peri-implantitis model [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 22-29. |
| [3] | Miao SHAO,Hui-fang GUO,Ling-yan LEI,Qing ZHAO,Yan-jie DING,Jin LIN,Rui WU,Feng YU,Yu-cui LI,Hua-li MIAO,Li-yun ZHANG,Yan DU,Rui-ying JIAO,Li-xia PANG,Li LONG,Zhan-guo LI,Ru LI. A multicenter study on the tolerance of intravenous low-dose cyclophosphamide in systemic lupus erythematosus [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1112-1116. |
| [4] | Lin ZHU,Wei-yu ZHANG,Ke-xin XU. Urodynamic and histological evaluation of cyclophosphamide-induced bladder pain syndrome in SD rats [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 735-740. |
| [5] | WANG Gui-hong,ZUO Ting,LI Ran,ZUO Zheng-cai. Effect of rebamipide on the acute gouty arthritis in rats induced by monosodium urate crystals [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 716-720. |
| [6] | WANG Jia-wen,LIU Jing-chao,MENG Ling-feng,ZHANG Wei,LIU Xiao-dong,ZHANG Yao-guang. Quality of life and related factors in patients with interstitial cystitis/bladder pain syndrome [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 653-658. |
| [7] | Tao WANG,Ke-xin XU,Wei-yu ZHANG,Hao HU,Xiao-wei ZHANG,Huan-rui WANG,Xian-hui LIU,Jing-wen CHEN,Xiao-peng ZHANG. Urodynamic classification of male patients with symptoms of overactive bladder and the outcome classification [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1048-1051. |
| [8] | Wei-yu ZHANG,Qiu-xiang XIA,Hao HU,Jing-wen CHEN,Yi-ran SUN,Ke-xin XU,Xiao-peng ZHANG. Analysis of urodynamic study of female outpatients with lower urinary tract symptoms and follow-up of the patients with detrusor underactive [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 856-862. |
| [9] | ZHANG Wei-yu, ZHANG Xiao-peng, CHEN Jing-wen, SUN Yi-ran, WANG Jia, HU Hao, XU Ke-xin. Effect of age on urodynamic parameters of women with urinary incontinence [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 825-829. |
| [10] | ZHANG Wei-yu, HU Hao, WANG Qi, CHEN Jing-wen, XU Ke-xin. Significance of preoperative urodynamics for clinical diagnosis of female patients with stress urinary incontinence [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 655-658. |
|
||