北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (4): 743-749. doi: 10.19723/j.issn.1671-167X.2020.04.028
牙龈卟啉单胞菌感染对载脂蛋白e基因敲除小鼠动脉粥样硬化的影响
轩艳1,蔡宇2,王啸轩2,石巧2,邱立新1,△( ),栾庆先2,△(
),栾庆先2,△( )
)
- 1.北京大学口腔医学院·口腔医院,第四门诊部,北京 100081
2.北京大学口腔医学院·口腔医院,牙周科 国家口腔疾病临床医学研究中心 口腔数字化医疗技术和材料国家工程实验室 口腔数字医学北京市重点实验室,北京 100081
Effect of Porphyromonas gingivalis infection on atherosclerosis in apolipoprotein-E knockout mice
Yan XUAN1,Yu CAI2,Xiao-xuan WANG2,Qiao SHI2,Li-xin QIU1,△( ),Qing-xian LUAN2,△(
),Qing-xian LUAN2,△( )
)
- 1. Fourth Clinical Division, Peking University School and Hospital of Stomatology, Beijing 100081, China
2. Department of Periodontology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
摘要:
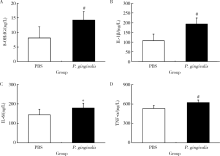
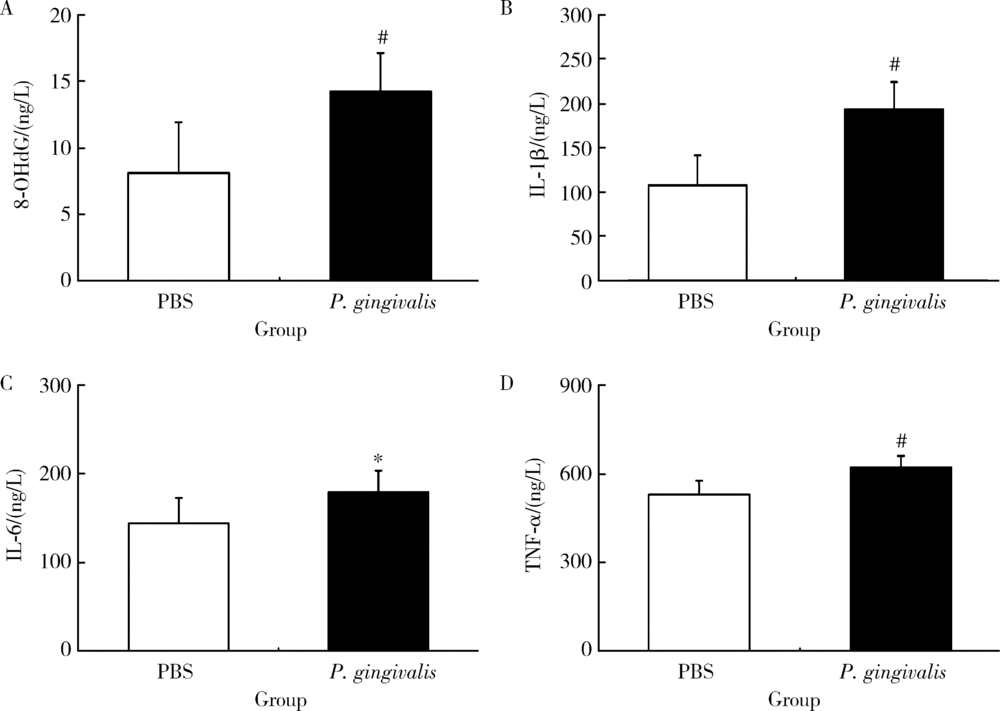
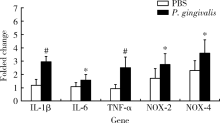
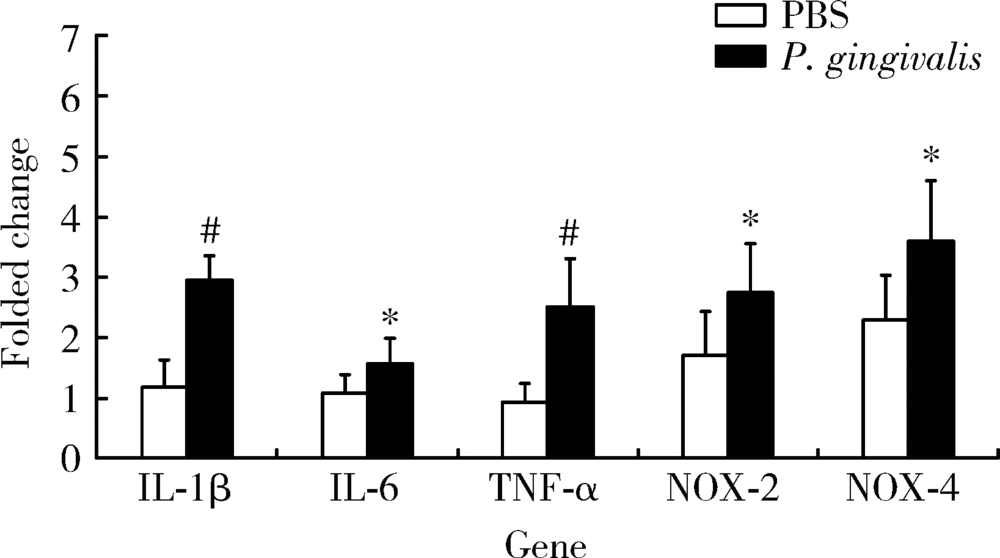
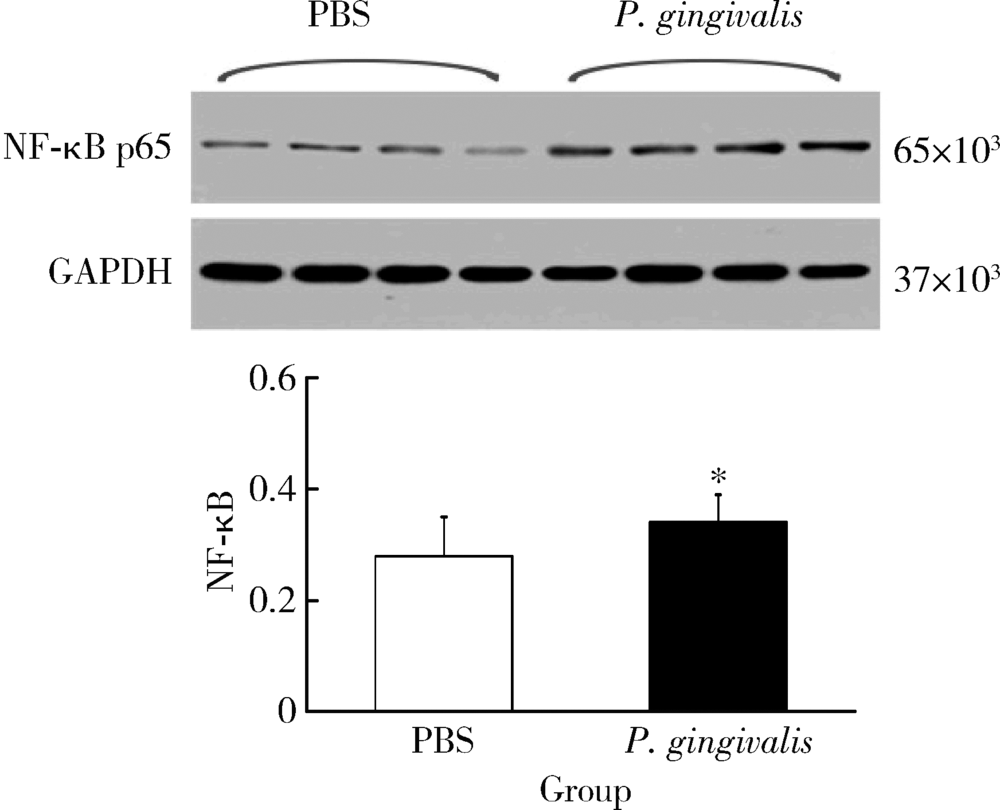
目的: 探讨牙龈卟啉单胞菌(Porphyromonas gingivalis,P. gingivalis)引起的炎症和氧化应激反应对动脉粥样硬化的影响及作用机制。方法: 采用8周龄载脂蛋白e基因敲除(ApoE knockout,ApoE-/-)小鼠建立动脉粥样硬化动物模型,将小鼠随机分为两组:(1)磷酸盐缓冲液(phosphate buffered saline,PBS)健康对照组:8只ApoE-/-小鼠,普通饮食+PBS鼠尾静脉注射;(2)P. gingivalis感染组:8只ApoE-/-小鼠,普通饮食+P. gingivalis鼠尾静脉注射。1周3次,隔天1次,共10次。4周后处死,取心脏组织进行油红O染色,血清进行酶联免疫吸附测定(enzyme-linked immunosorbent assay,ELISA),主动脉进行实时荧光定量PCR以及Western blot检测。结果: P. gingivalis 感染组较PBS健康对照组可以显著加重ApoE-/-小鼠动脉粥样硬化斑块的形成,增加血清中炎症介质,如白细胞介素(interleukin,IL)-1β、IL-6和肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)以及氧化应激介质8-羟脱氧鸟苷(8-hydroxy-2-deoxyguanosine,8-OHDG)表达,增加主动脉组织中IL-1β、IL-6、TNF-α、NADPH 氧化酶(NADPH oxidase,NOX)-2和NOX-4基因的mRNA水平。P. gingivalis感染后在主动脉组织中观察到核转录因子-κB(nuclear factor-kappa B,NF-κB)表达有增高趋势。结论: P.gingivalis感染会加速ApoE-/-小鼠动脉粥样硬化进程,诱导氧化应激和炎症反应;NF-κB信号通路可能是P. gingivalis加速动脉粥样硬化形成的重要作用机制。
中图分类号:
- R781.42
| [1] | Cutler CW, Kalmar JR, Genco CA. Pathogenic strategies of the oral anaerobe, Porphyromonas gingivalis[J]. Trends Microbiol, 1995,3(2):45-51. |
| [2] | Holt SC, Kesavalu L, Walker S, et al. Virulence factors of Porphyromonas gingivalis[J]. Periodontol 2000,1999(20):168-238. |
| [3] | DeStefano F, Anda RF, Kahn HS, et al. Dental disease and risk of coronary heart disease and mortality[J]. BMJ, 1993,306(6879):688-691. |
| [4] | Southerland JH, Taylor GW, Moss K, et al. Commonality in chronic inflammatory diseases: Periodontitis, diabetes, and coronary artery disease[J]. Periodontol 2000, 2006,40(1):130-143. |
| [5] |
Dogan B, Buduneli E, Emingil G, et al. Characteristics of periodontal microflora in acute myocardial infarction[J]. J Periodontol, 2005,76(5):740-748.
pmid: 15898935 |
| [6] |
Jain A, Batista EL Jr, Serhan C, et al. Role for periodontitis in the progression of lipid deposition in an animal model[J]. Infect Immun, 2003,71(10):6012-6018.
doi: 10.1128/iai.71.10.6012-6018.2003 pmid: 14500522 |
| [7] | Ishihara K, Nabuchi A, Ito R, et al. Correlation between detection rates of periodontopathic bacterial DNA in carotid coronary stenotic artery plaque and in dental plaque samples[J]. J Clin Microbiol, 2004,42(3):1313-1315. |
| [8] | Nakano K, Inaba H, Nomura R, et al. Distribution of Porphyromonas gingivalis fimA genotypes in cardiovascular specimens from Japanese patients[J]. Oral Microbiol Immunol, 2008,23(2):170-172. |
| [9] | Kozarov EV, Dorn BR, Shelburne CE, et al. Human atherosclerotic plaque contains viable invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis[J]. Arterioscler Thromb Vasc Biol, 2005,25(3):e17-e18. |
| [10] |
Fukasawa A, Kurita-Ochiai T, Hashizume T, et al. Porphyromonas gingivalis accelerates atherosclerosis in C57BL/6 mice fed a high-fat diet[J]. Immunopharmacol Immunotoxicol, 2012,34(3):470-476.
doi: 10.3109/08923973.2011.627866 pmid: 22047042 |
| [11] |
Yu H, Qi LT, Liu LS, et al. Association of carotid intima-media thickness and atherosclerotic plaque with periodontal status[J]. J Dent Res, 2014,93(8):744-751.
doi: 10.1177/0022034514538973 pmid: 24935064 |
| [12] | Lin G, Chen S, Lei L, et al. Effects of intravenous injection of Porphyromonas gingivalis on rabbit inflammatory immune response and atherosclerosis [J/OL]. Mediators Inflamm, 2015: 364391. doi: 10.1155/2015/364391. |
| [13] |
Paigen B, Morrow A, Holmes PA, et al. Quantitative assessment of atherosclerotic lesions in mice[J]. Atherosclerosis, 1987,68(3):231-240.
pmid: 3426656 |
| [14] | Bélanger M, Rodrigues PH, Dunn WA, et al. Autophagy: A highway for Porphyromonas gingivalis in endothelial cells[J]. Autophagy, 2006,2(3):165-170. |
| [15] | Iwai T. Periodontal bacteremia and various vascular diseases[J]. J Periodontal Res, 2009,44(6):689-694. |
| [16] |
Campbell LA, Rosenfeld ME. Infection and atherosclerosis deve-lopment[J]. Arch Med Res, 2015,46(5):339-350.
doi: 10.1016/j.arcmed.2015.05.006 pmid: 26004263 |
| [17] |
Mattila KJ, Nieminen MS, Valtonen VV, et al. Association between dental health and acute myocardial infarction[J]. BMJ, 1989,298(6676):779-781.
doi: 10.1136/bmj.298.6676.779 pmid: 2496855 |
| [18] |
Chiu B. Multiple infections in carotid atherosclerotic plaques[J]. Am Heart J, 1999,138(5 Pt 2):S534-S536.
pmid: 10539867 |
| [19] | Haraszthy VI, Zambon JJ, Trevisan M, et al. Identification of periodontal pathogens in atheromatous plaques[J]. J Periodontol, 2000,71(10):1554-1560. |
| [20] |
Nakano K, Miyamoto E, Tamura K, et al. Distribution of 10 periodontal bacterial species in children and adolescents over a 7-year period[J]. Oral Dis, 2008,14(7):658-664.
doi: 10.1111/j.1601-0825.2008.01452.x pmid: 18565147 |
| [21] |
Wada K, Kamisaki Y. Roles of oral bacteria in cardiovascular diseases. From molecular mechanisms to clinical cases: Involvement of Porphyromonas gingivalis in the development of human aortic aneurysm[J]. J Pharmacol Sci, 2010,113(2):115-119.
doi: 10.1254/jphs.09r22fm pmid: 20501967 |
| [22] | Miyakawa H, Honma K, Qi M, et al. Interaction of Porphyromonas gingivalis with low-density lipoproteins: implications for a role for periodontitis in atherosclerosis[J]. J Periodontal Res, 2004,39(1):1-9. |
| [23] |
Li XY, Wang C, Xiang XR, et al. Porphyromonas gingivalis lipopolysaccharide increases lipid accumulation by affecting CD36 and ATP-binding cassette transporter A1 in macrophages[J]. Oncol Rep, 2013,30(3):1329-1336.
pmid: 23835648 |
| [24] |
Qi M, Miyakawa H, Kuramitsu HK. Porphyromonas gingivalis induces murine macrophage foam cell formation[J]. Microb Pathog, 2003,35(6):259-267.
pmid: 14580389 |
| [25] | Ross R. Atherosclerosis is an inflammatory disease[J]. Am Heart J, 1999,138(5 Pt 2):S419-S420. |
| [26] |
Hansson GK. Inflammation and immune response in atherosclerosis[J]. Curr Atheroscler Rep, 1999,1(2):150-155.
pmid: 11122704 |
| [27] |
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis[J]. Nature, 2011,473(7347):317-325.
pmid: 21593864 |
| [28] |
Ramji DP, Davies TS. Cytokines in atherosclerosis: Key players in all stages of disease and promising therapeutic targets[J]. Cytokine Growth Factor Rev, 2015,26(6):673-685.
doi: 10.1016/j.cytogfr.2015.04.003 pmid: 26005197 |
| [29] |
Rosenson RS, Elliott M, Stasiv Y, et al. Randomized trial of an inhibitor of secretory phospholipase A2 on atherogenic lipoprotein subclasses in statin-treated patients with coronary heart disease[J]. Eur Heart J, 2011,32(8):999-1005.
doi: 10.1093/eurheartj/ehq374 pmid: 21081550 |
| [30] |
Hayashi C, Papadopoulos G, Gudino CV, et al. Protective role for TLR4 signaling in atherosclerosis progression as revealed by infection with a common oral pathogen[J]. J Immunol, 2012,189(7):3681-3688.
doi: 10.4049/jimmunol.1201541 pmid: 22956579 |
| [31] | Gibson FC 3rd, Ukai T, Genco CA. Engagement of specific innate immune signaling pathways during Porphyromonas gingivalis induced chronic inflammation and atherosclerosis[J]. Front Biosci, 2008(13):2041-2059. |
| [32] |
Pan S, Lei L, Chen S, et al. Rosiglitazone impedes Porphyromonas gingivalis-accelerated atherosclerosis by downregulating the TLR/NF-κB signaling pathway in atherosclerotic mice[J]. Int Immunopharmacol, 2014,23(2):701-708.
doi: 10.1016/j.intimp.2014.10.026 pmid: 25445963 |
| [33] |
Huang CY, Shih CM, Tsao NW, et al. The GroEL protein of Porphyromonas gingivalis regulates atherogenic phenomena in endothelial cells mediated by up-regulating toll-like receptor 4 expression[J]. Am J Transl Res, 2016,8(2):384-404.
pmid: 27158334 |
| [34] |
Khlgatian M, Nassar H, Chou HH, et al. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells[J]. Infect Immun, 2002,70(1):257-267.
doi: 10.1128/iai.70.1.257-267.2002 pmid: 11748191 |
| [35] |
Gitlin JM, Loftin CD. Cyclooxygenase-2 inhibition increases lipopolysaccharide-induced atherosclerosis in mice[J]. Cardiovasc Res, 2009,81(2):400-407.
doi: 10.1093/cvr/cvn286 pmid: 18948273 |
| [36] |
Huang KT, Kuo L, Liao JC. Lipopolysaccharide activates endothelial nitric oxide synthase through protein tyrosine kinase[J]. Biochem Biophys Res Commun, 1998,245(1):33-37.
doi: 10.1006/bbrc.1998.8384 pmid: 9535778 |
| [37] |
Obermeier F, Gross V, Scholmerich J, et al. Interleukin-1 production by mouse macrophages is regulated in a feedback fashion by nitric oxide[J]. J Leukoc Biol, 1999,66(5):829-836.
pmid: 10577516 |
| [38] |
Sorescu D, Weiss D, Lassègue B. Superoxide production and expression of nox family proteins in human atherosclerosis[J]. Circulation, 2002,105(12):1429-1435.
doi: 10.1161/01.cir.0000012917.74432.66 pmid: 11914250 |
| [39] |
Szöcs K, Lassègue B, Sorescu D, et al. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury[J]. Arterioscler Thromb Vasc Biol, 2002,22(1):21-27.
doi: 10.1161/hq0102.102189 pmid: 11788456 |
| [40] |
Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates pha-gocytic NADPH oxidase by inducing the expression of gp91phox[J]. J Biol Chem, 2006,281(9):5657-5667.
doi: 10.1074/jbc.M506172200 pmid: 16407283 |
| [41] |
Morris KR, Lutz RD, Choi HS, et al. Role of the NF-kappaB signaling pathway and kappaB cis-regulatory elements on the IRF-1 and iNOS promoter regions in mycobacterial lipoarabinomannan induction of nitric oxide[J]. Infect Immun, 2003,71(3):1442-1452.
doi: 10.1128/iai.71.3.1442-1452.2003 pmid: 12595462 |
| [42] |
Deng WG, Zhu Y, Wu KK. Up-regulation of p300 binding and p50 acetylation in tumor necrosis factor-alpha-induced cyclooxygenase-2 promoter activation[J]. J Biol Chem, 2003,278(7):4770-4777.
doi: 10.1074/jbc.M209286200 pmid: 12471036 |
| [43] |
Brown K, Gerstberger S, Carlson L, et al. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation[J]. Science, 1995,267(5203):1485-1488.
doi: 10.1126/science.7878466 pmid: 7878466 |
| [44] |
Luoma JS, Stralin P, Marklund SL, et al. Expression of extracellular SOD and iNOS in macrophages and smooth muscle cells in human and rabbit atherosclerotic lesions: colocalization with epitopes characteristic of oxidized LDL and peroxynitrite-modified proteins[J]. Arterioscler Thromb Vasc Biol, 1998,18(2):157-167.
doi: 10.1161/01.atv.18.2.157 pmid: 9484979 |
| [45] |
Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappa B signaling[J]. Cell Res, 2011,21(1):103-115.
doi: 10.1038/cr.2010.178 pmid: 21187859 |
| [46] |
Stocker R, Keaney JF Jr. Role of oxidative modifications in atherosclerosis[J]. Physiol Rev, 2004,84(4):1381-1478.
doi: 10.1152/physrev.00047.2003 pmid: 15383655 |
| [1] | 和静,房中则,杨颖,刘静,马文瑶,霍勇,高炜,武阳丰,谢高强. 血浆中脂质代谢分子与颈动脉粥样硬化斑块、传统心血管危险因素及膳食因素的关系[J]. 北京大学学报(医学版), 2024, 56(4): 722-728. |
| [2] | 马会超,李军,王永清. 妊娠合并炎症性肠病的临床特点[J]. 北京大学学报(医学版), 2024, 56(2): 260-266. |
| [3] | 俞光岩. 儿童唾液腺疾病[J]. 北京大学学报(医学版), 2024, 56(1): 1-3. |
| [4] | 殳畅,韩烨,孙雨哲,杨再目,侯建霞. Ⅲ期牙周炎患者牙周基础治疗前后炎症性贫血相关指标的变化[J]. 北京大学学报(医学版), 2024, 56(1): 45-50. |
| [5] | 刘志伟,刘鹏,孟凡星,李天水,王颖,高嘉琪,周佐邑,王聪,赵斌. 内源性二氧化硫对脓毒症大鼠心肌氧化应激的调节[J]. 北京大学学报(医学版), 2023, 55(4): 582-586. |
| [6] | 刘颖,霍然,徐慧敏,王筝,王涛,袁慧书. 磁共振血管壁成像评估颈动脉中重度狭窄患者斑块特征与脑血流灌注的相关性[J]. 北京大学学报(医学版), 2023, 55(4): 646-651. |
| [7] | 梁秀睿,闪雪纯,关晶,张锐,杨静,张怡,金佳琦,张誉馨,徐凡,傅继华. 高血糖诱导肝星状细胞5-羟色胺降解在2型糖尿病致肝脏炎症和纤维化时的作用[J]. 北京大学学报(医学版), 2022, 54(6): 1141-1150. |
| [8] | 贺冰洁,刘志科,沈鹏,孙烨祥,陈彬,詹思延,林鸿波. 2011—2020年宁波市鄞州区炎症性肠病发病的流行病学研究[J]. 北京大学学报(医学版), 2022, 54(3): 511-519. |
| [9] | 郭辅政,赵秀娟,邓玖旭,杜哲,王天兵,朱凤雪. 严重创伤患者早期外周血淋巴细胞变化与预后之间的关系[J]. 北京大学学报(医学版), 2022, 54(3): 552-556. |
| [10] | 王向熙,李臻臻,赖彦云,杨莉,史霖丽,仲少敏,吴艳. 585 nm Q开关激光治疗痤疮炎症性皮损和炎症后红斑的疗效[J]. 北京大学学报(医学版), 2022, 54(2): 283-288. |
| [11] | 袁临天,马利沙,刘润园,齐伟,张栌丹,王贵燕,王宇光. 计算机模拟亚甲基蓝与牙龈卟啉单胞菌部分蛋白的分子对接[J]. 北京大学学报(医学版), 2022, 54(1): 23-30. |
| [12] | 伊文霞,魏翠洁,吴晔,包新华,熊晖,常杏芝. 长疗程利妥昔单抗治疗难治性幼年型特发性炎症性肌病3例[J]. 北京大学学报(医学版), 2021, 53(6): 1191-1195. |
| [13] | 白枫,何倚帆,牛亚楠,杨若娟,曹静. 超细颗粒物对大鼠离体灌注心脏功能的影响[J]. 北京大学学报(医学版), 2021, 53(2): 240-245. |
| [14] | 陈怀安,刘硕,李秀君,王哲,张潮,李凤岐,苗文隆. 炎症生物标志物对输尿管尿路上皮癌患者预后预测的临床价值[J]. 北京大学学报(医学版), 2021, 53(2): 302-307. |
| [15] | 杨林承,张瑞涛,郭丽君,肖晗,祖凌云,张幼怡,程秦,赵志伶,葛庆岗,高炜. 低氧状态及炎症反应是新型冠状病毒肺炎患者发生急性心肌损伤的危险因素[J]. 北京大学学报(医学版), 2021, 53(1): 159-166. |
|
||